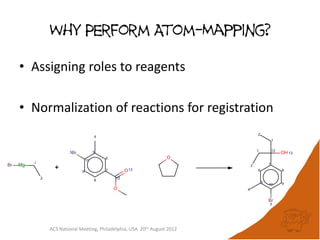
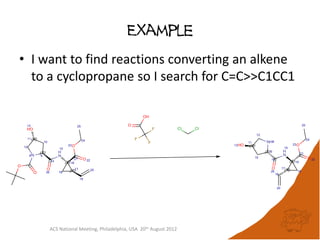
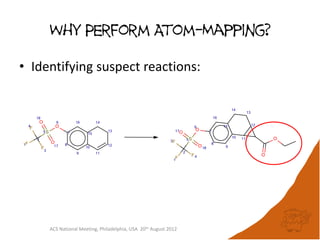
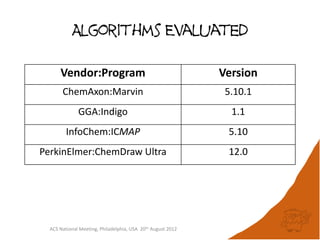
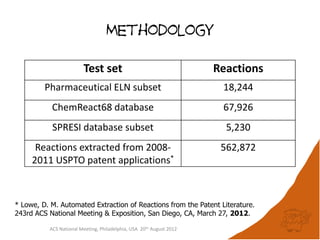
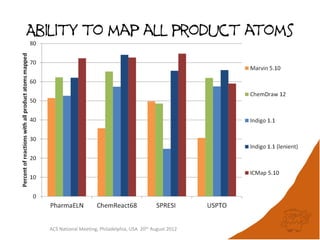
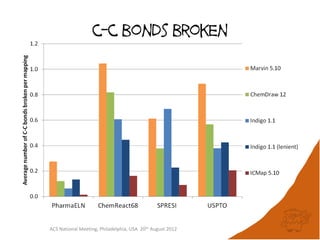
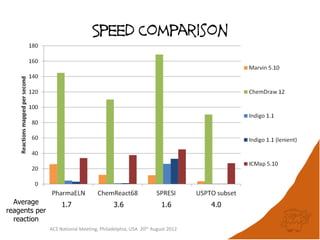
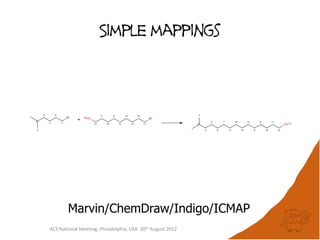
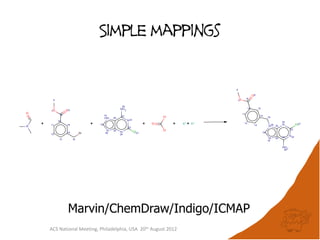
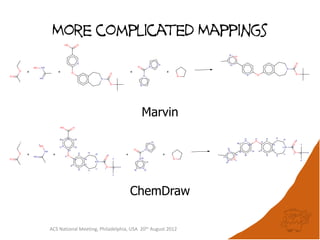
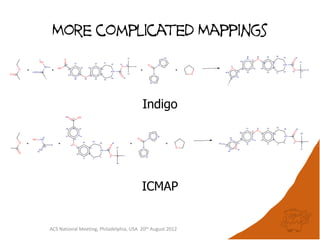
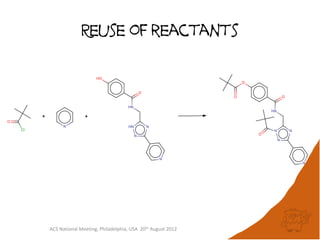
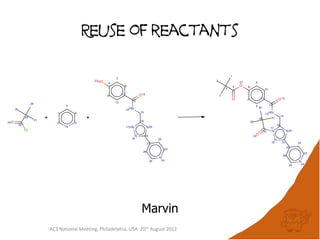
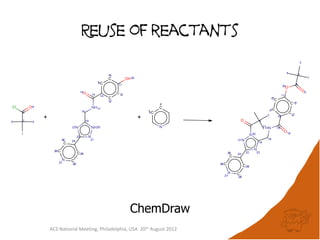
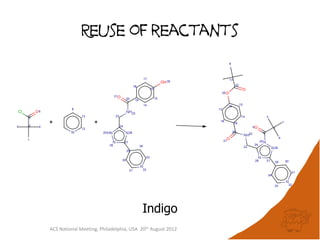
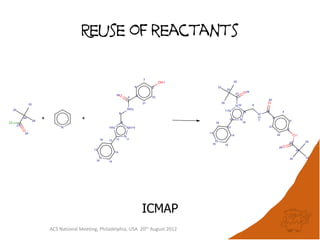
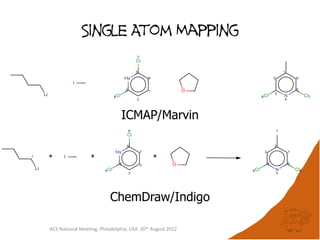
The document evaluates the quality and performance of various atom mapping algorithms, highlighting their importance for precise database searches and reaction analysis. It details methodologies, test sets, and algorithms used, such as those from ChemAxon and PerkinElmer, and discusses their capabilities and shortcomings. The conclusions suggest that icmap yields the best quality mappings, while all algorithms have areas for improvement.