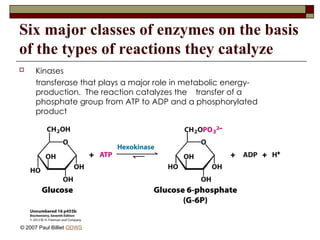
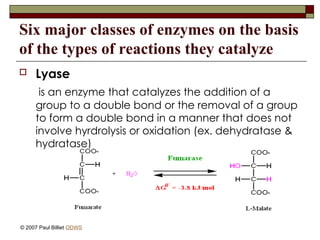
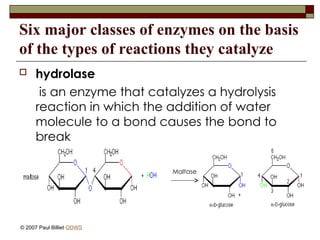
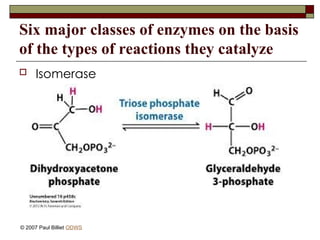
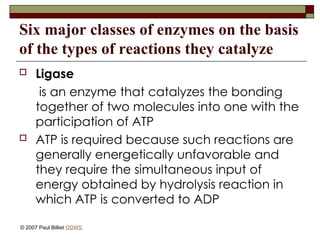
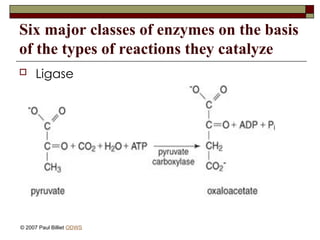
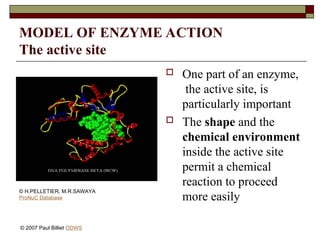
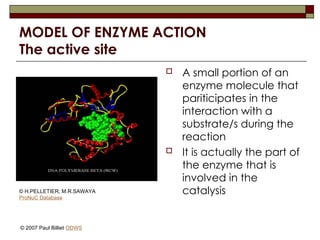
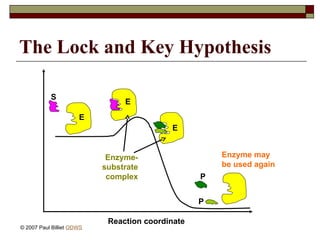
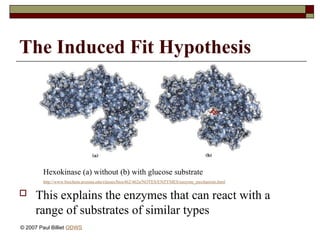
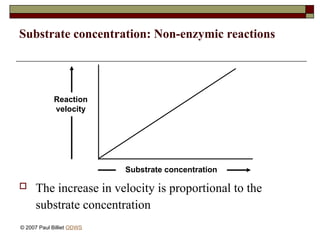
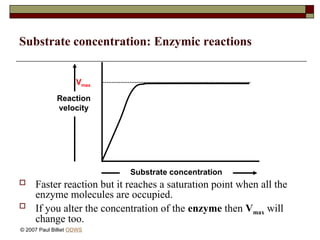
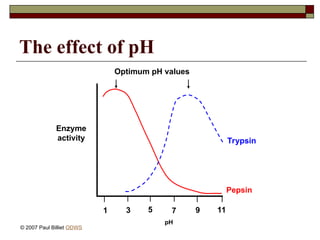
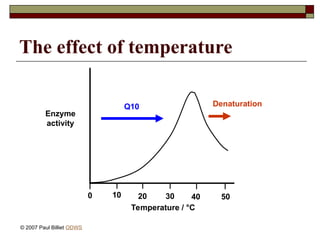
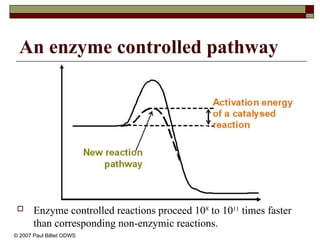
aasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxnaasdsahxugjhbxn