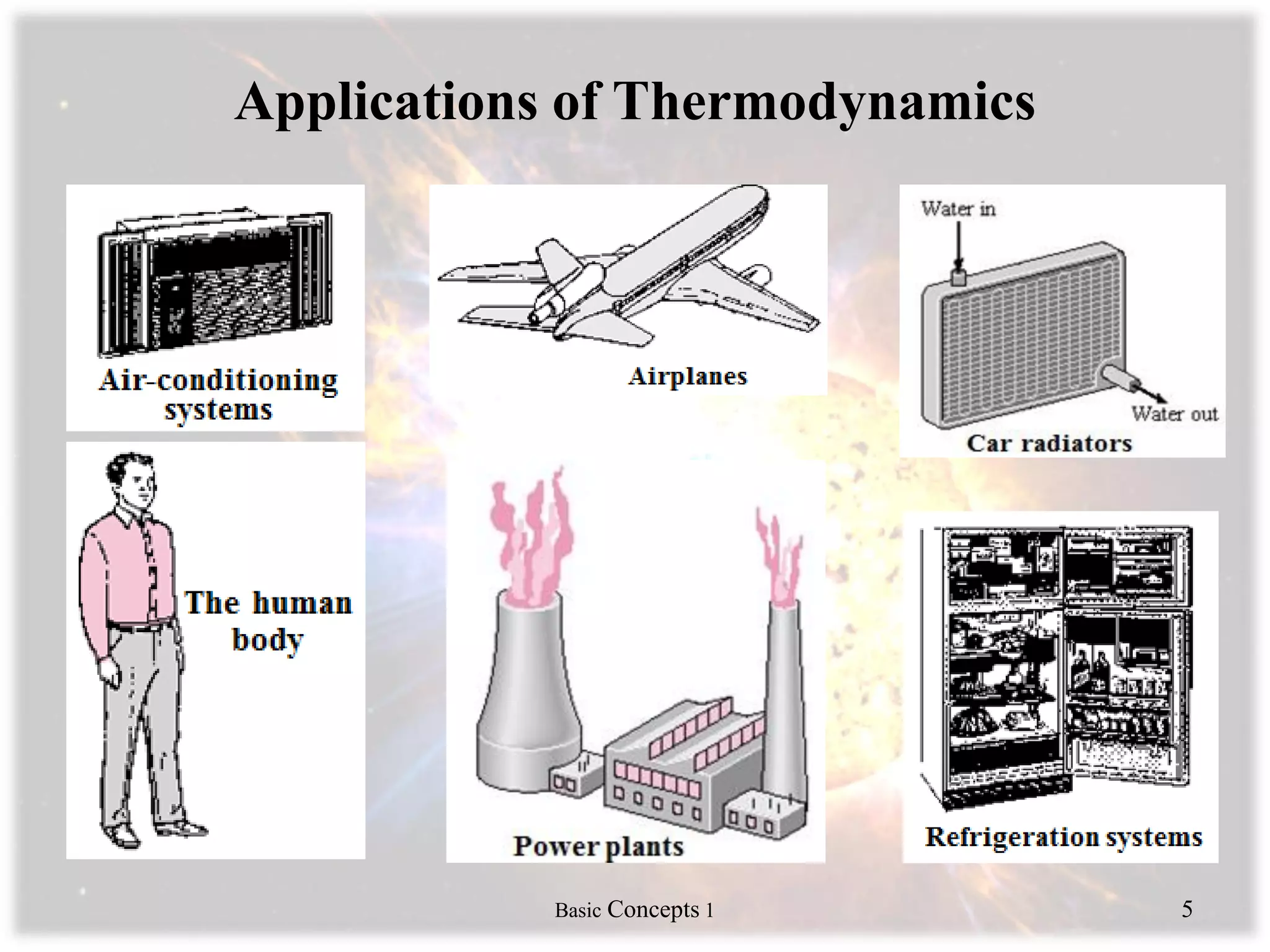
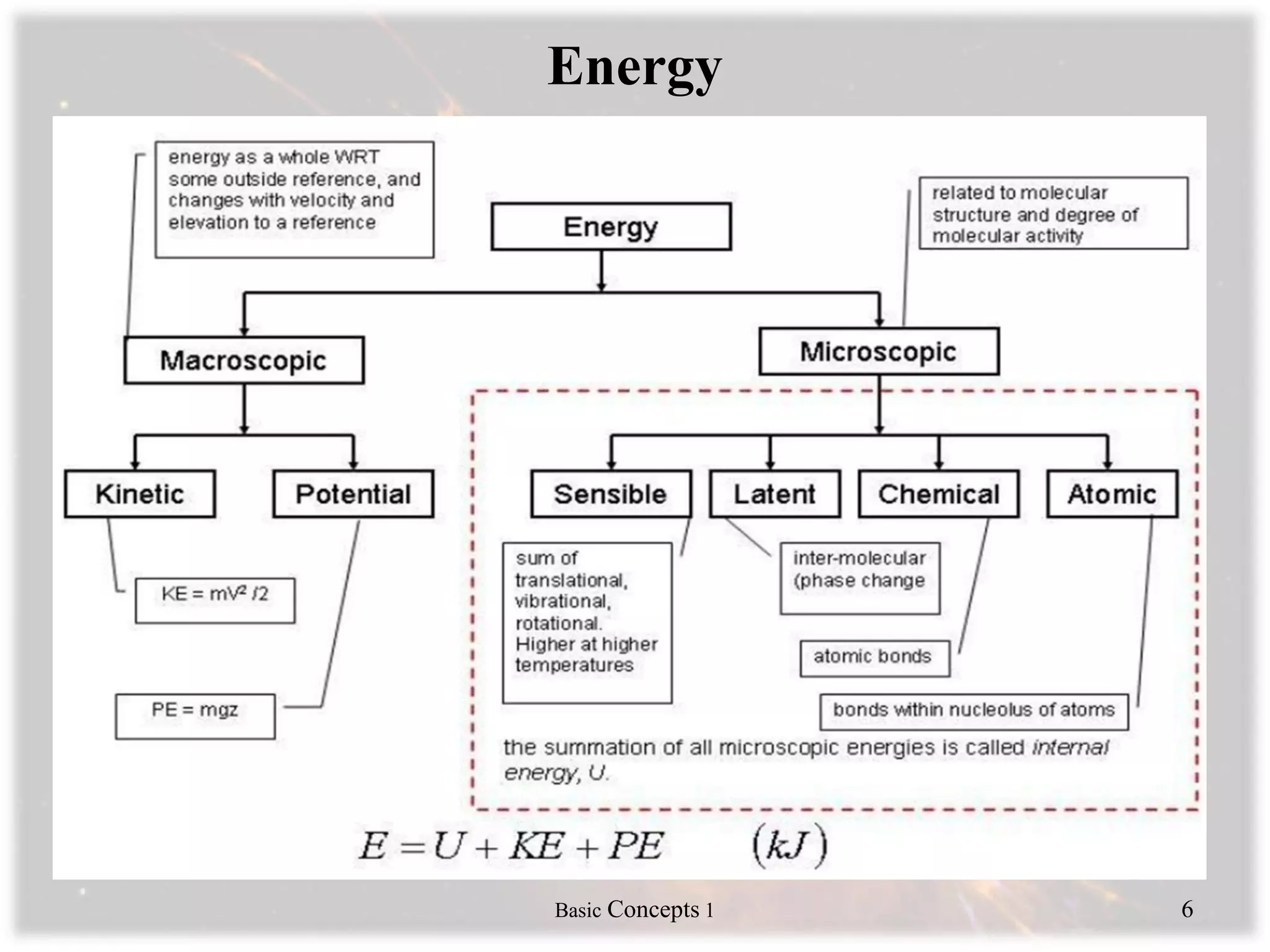
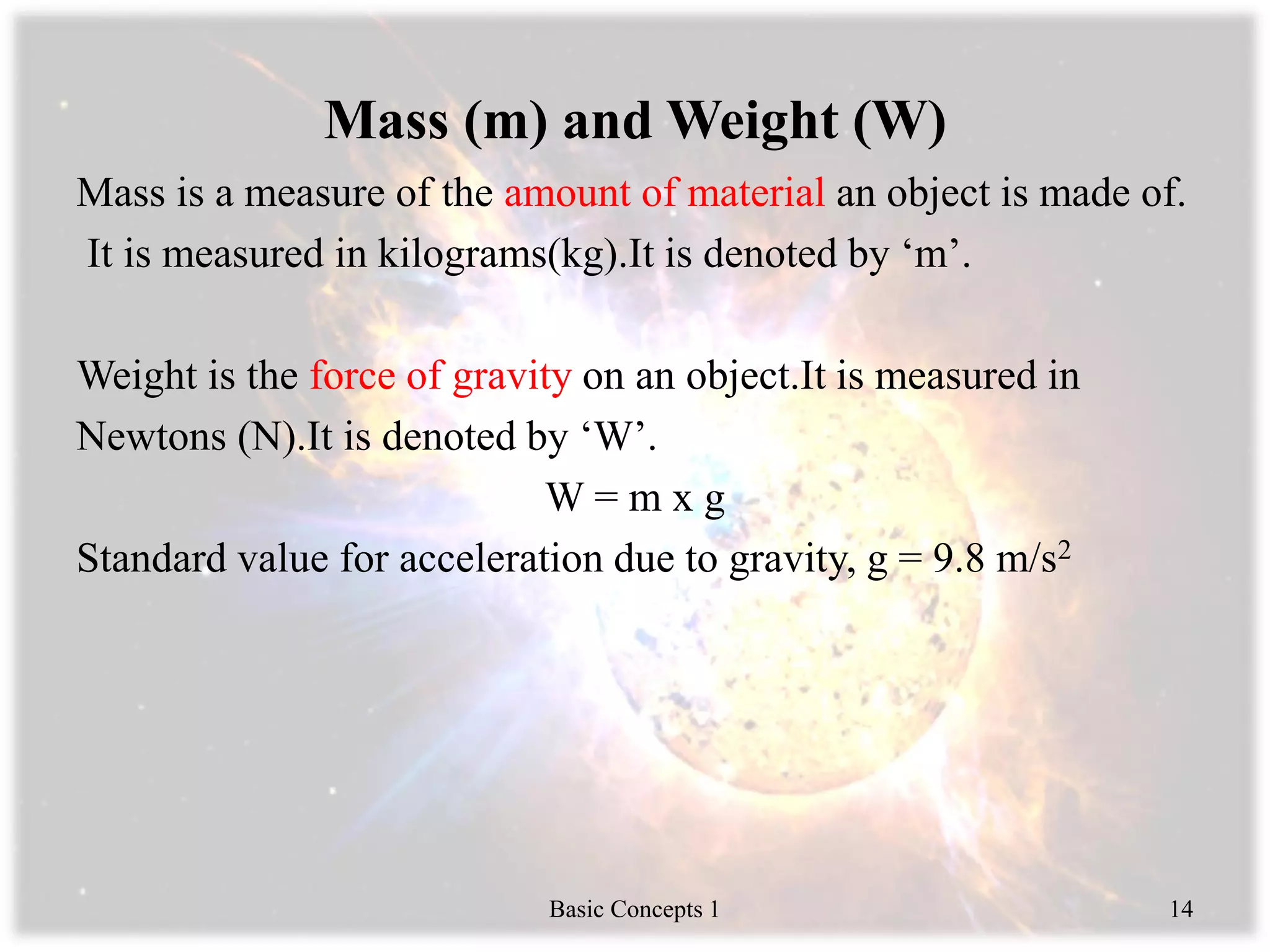
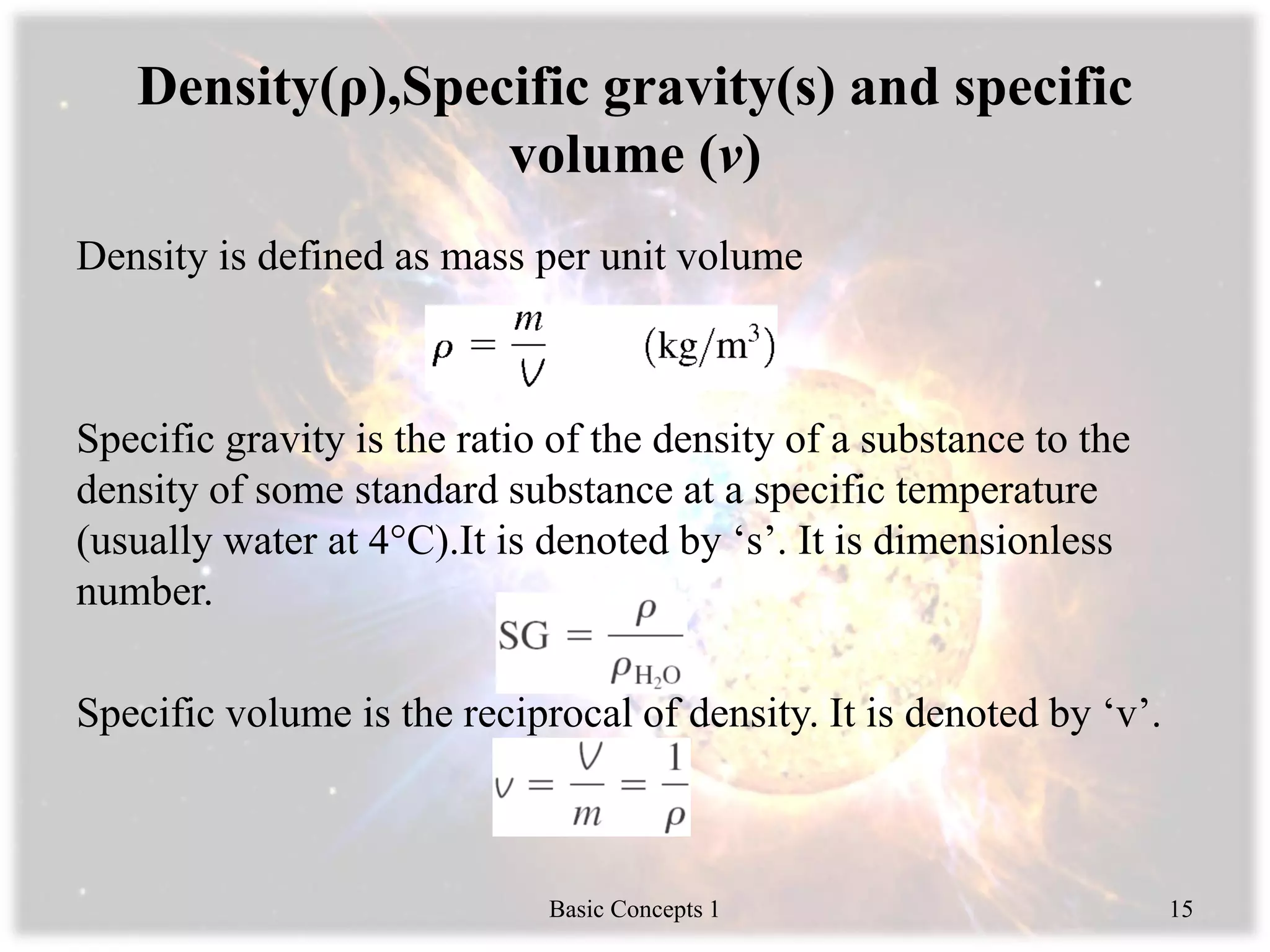
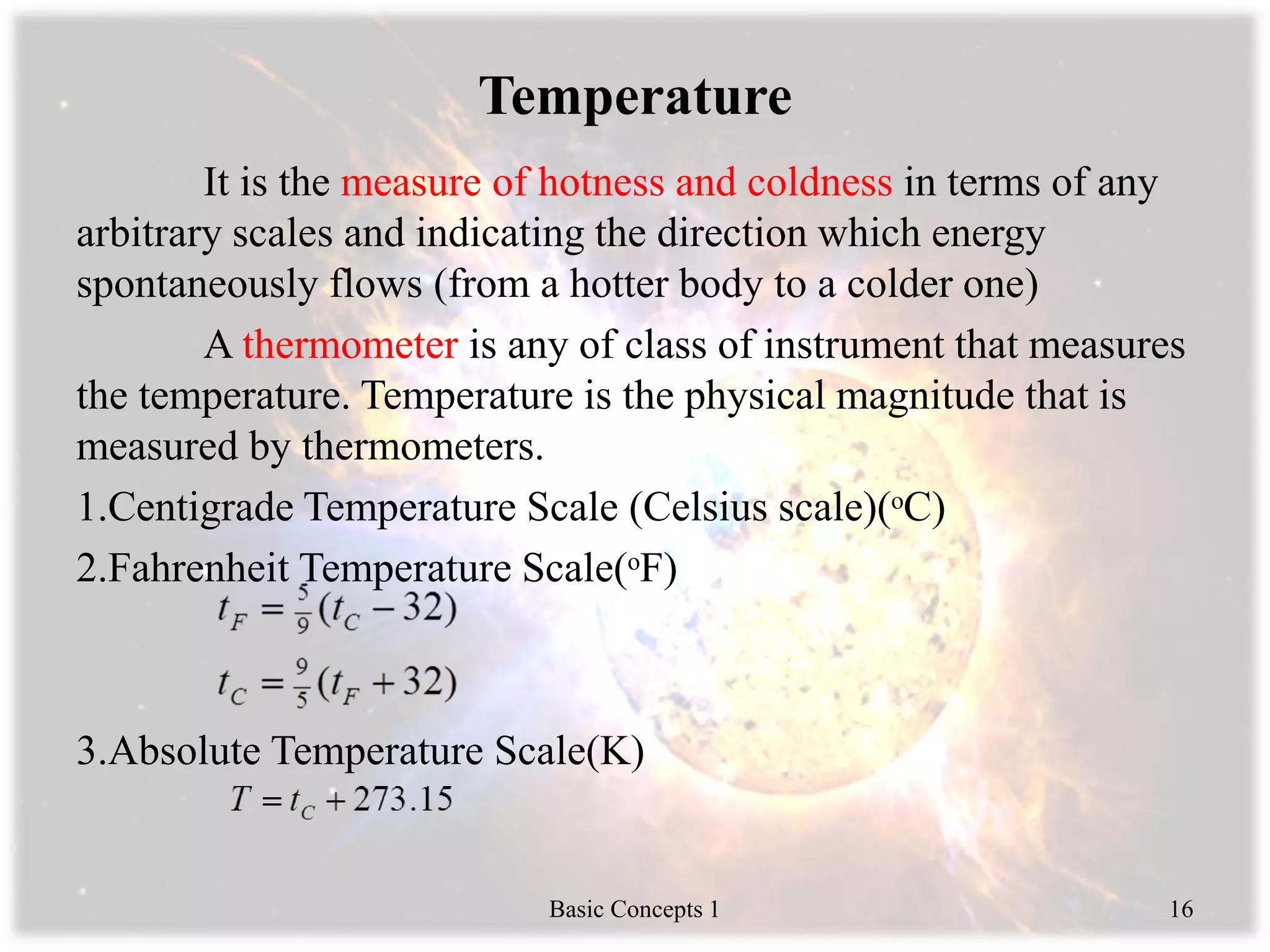
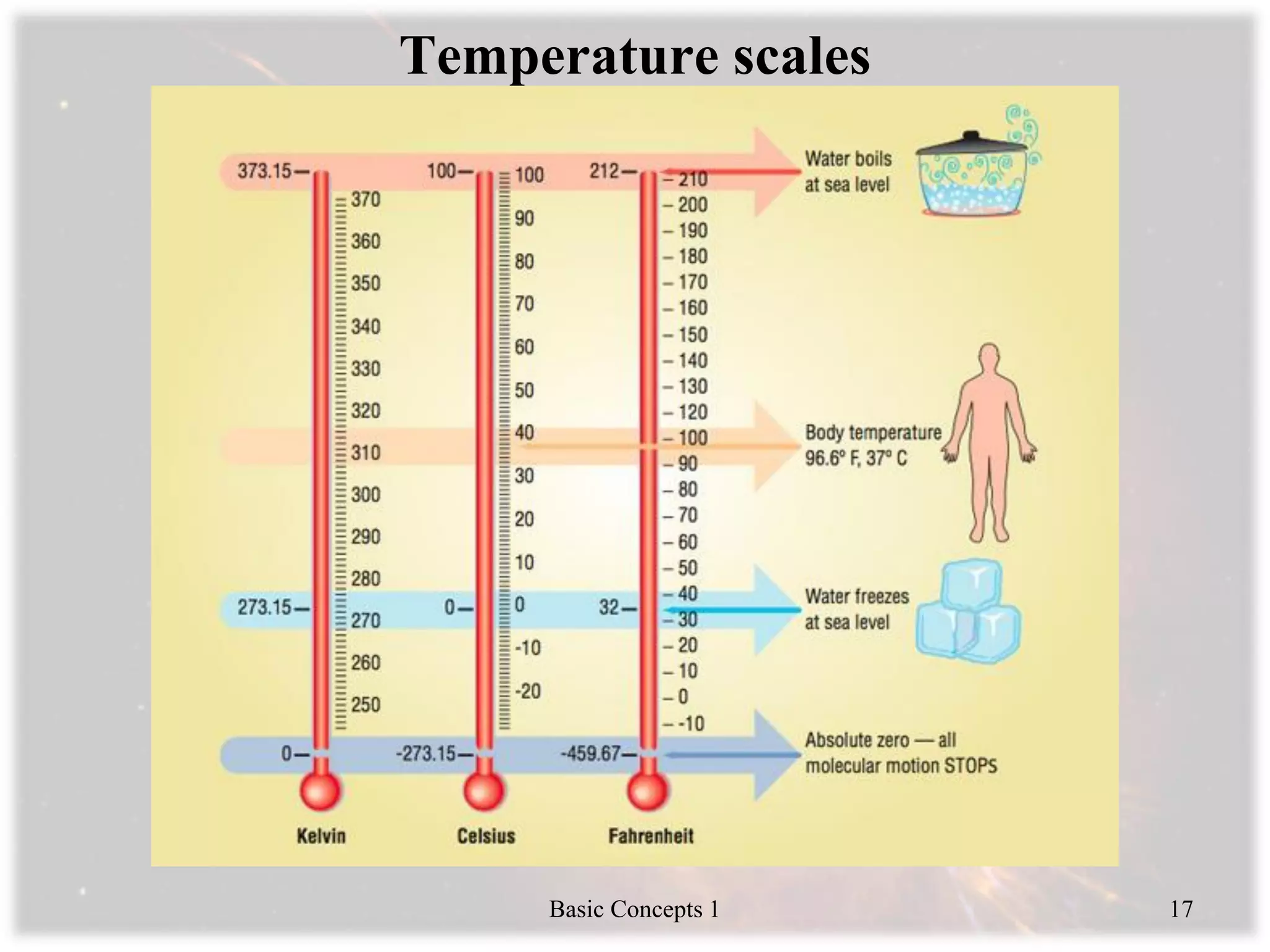
Engineering thermodynamics covers basic concepts including:
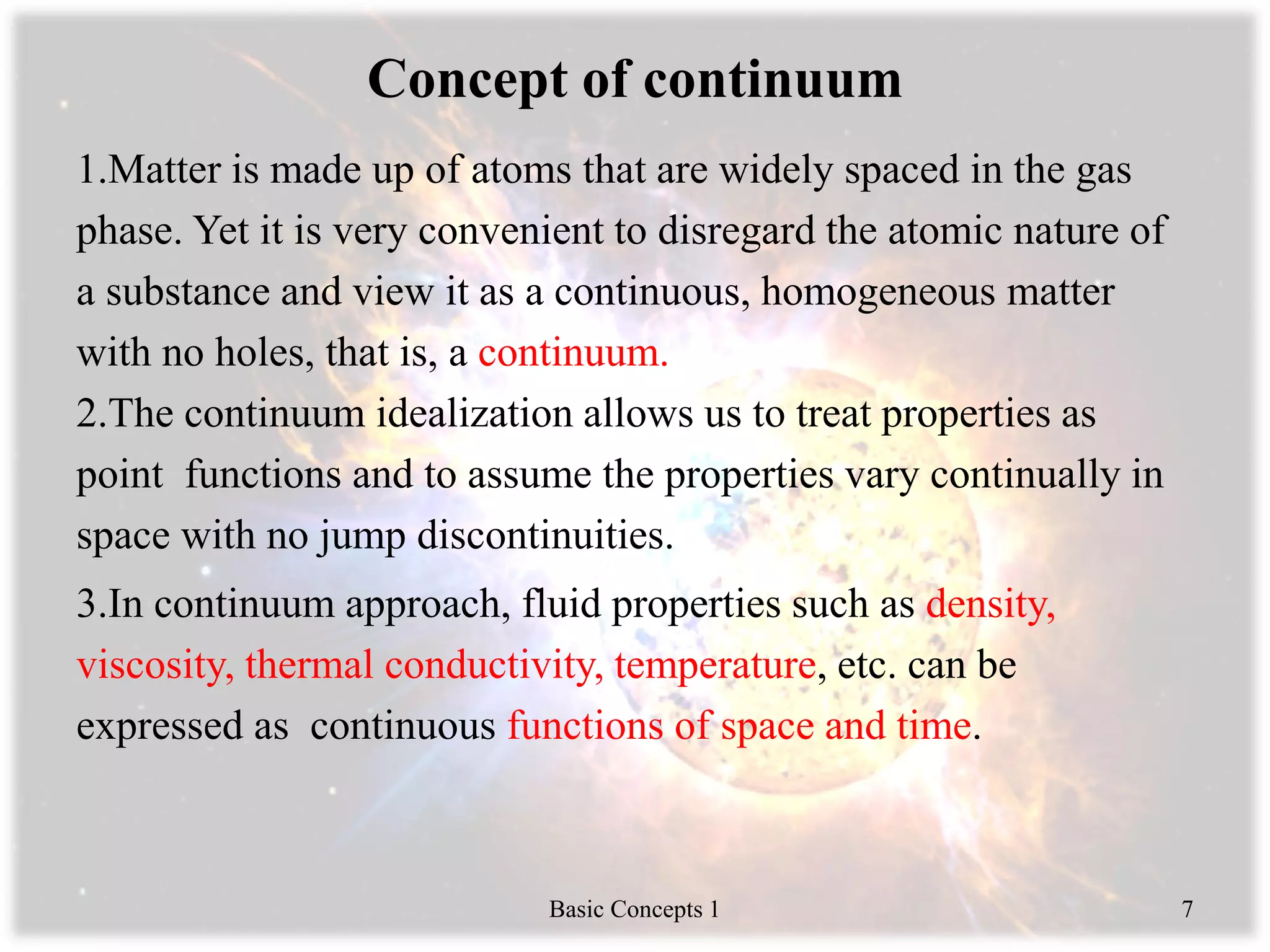
1) The continuum approach views matter as a continuous substance rather than discrete atoms, allowing properties to vary continuously through space.
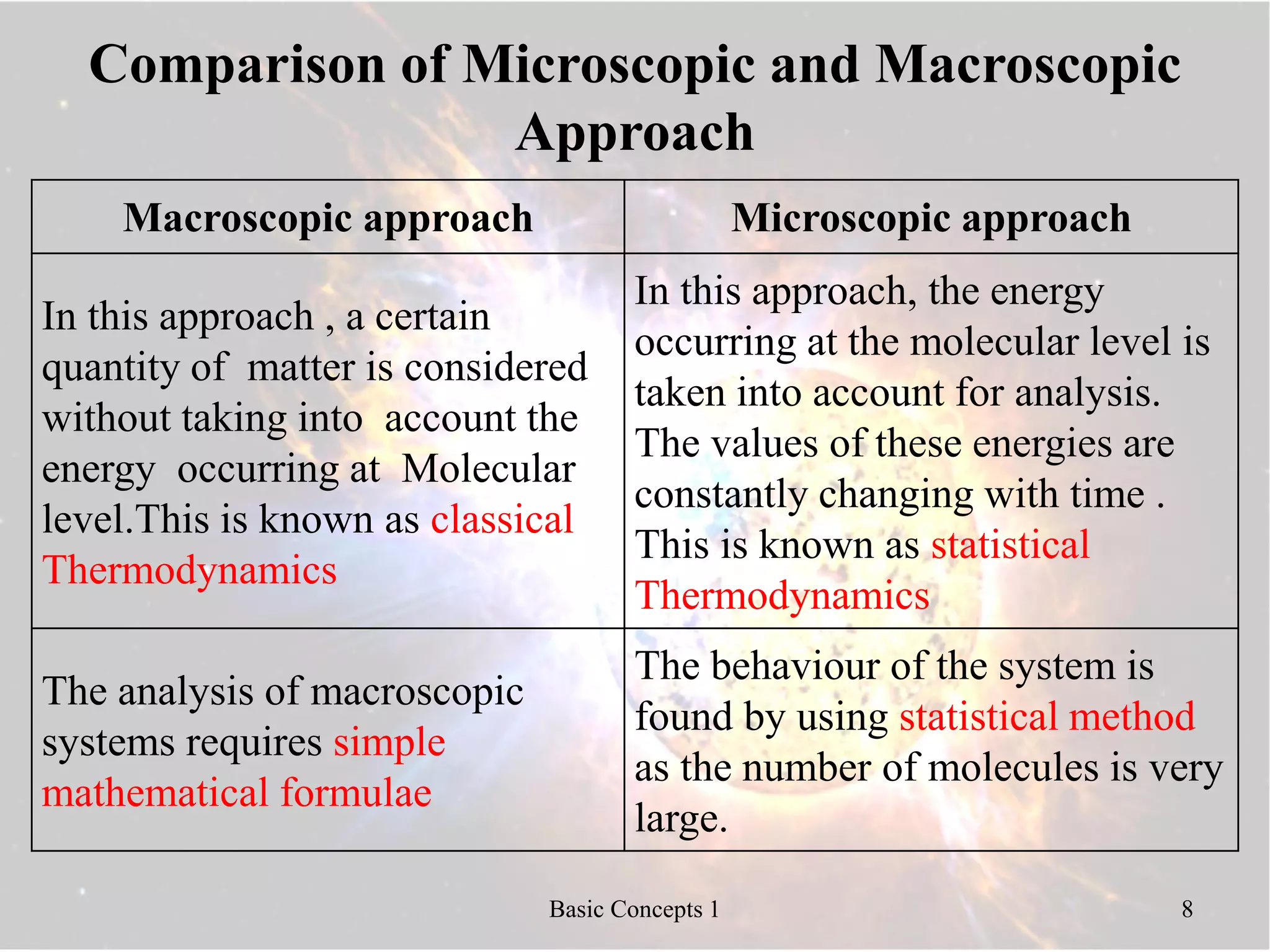
2) Macroscopic and microscopic approaches differ in whether properties are averaged or describe individual molecules.
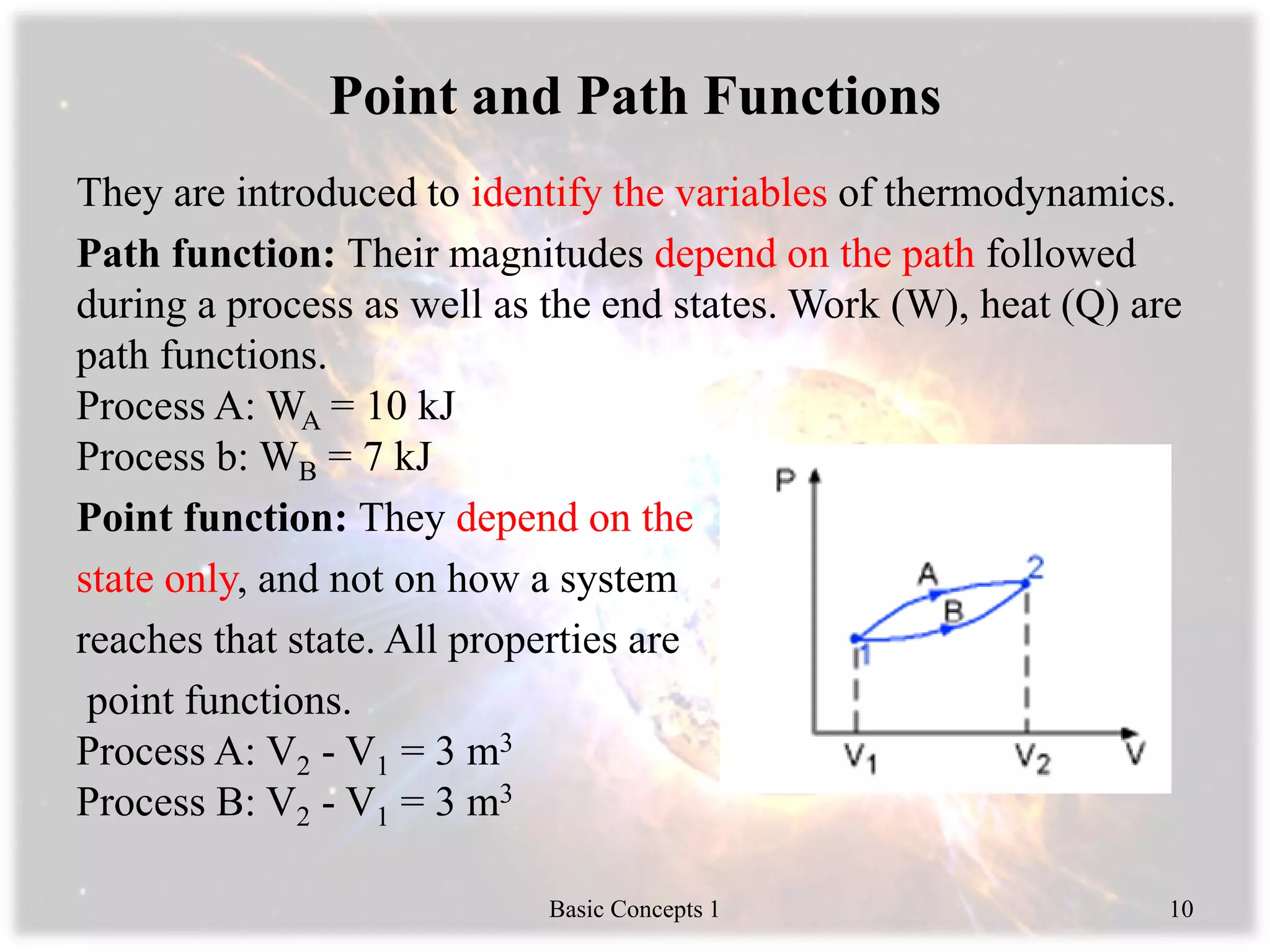
3) Path functions like work and heat depend on process, while point functions like volume depend only on state.
4) Intensive properties are independent of system size, while extensive properties depend on size.