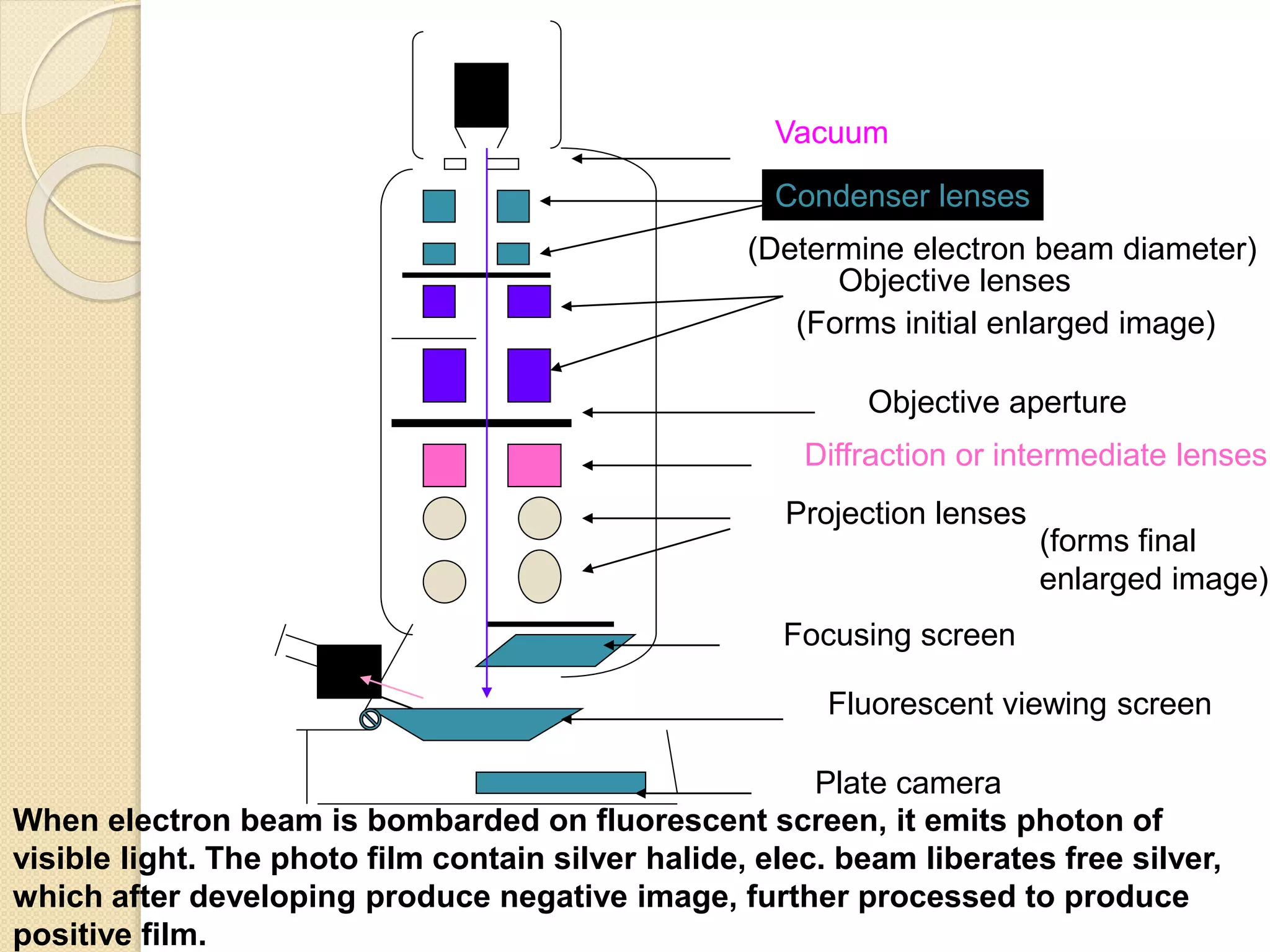
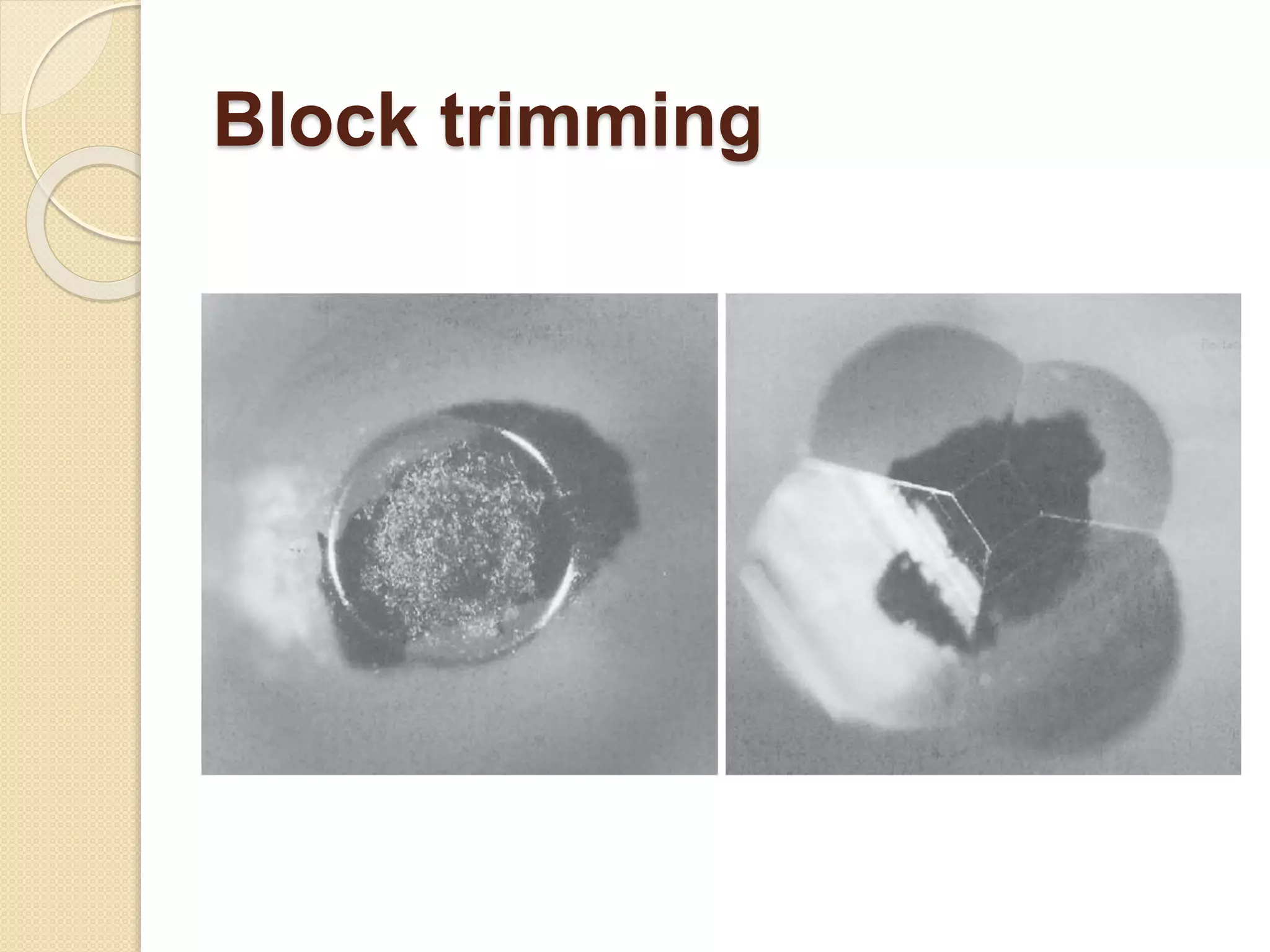
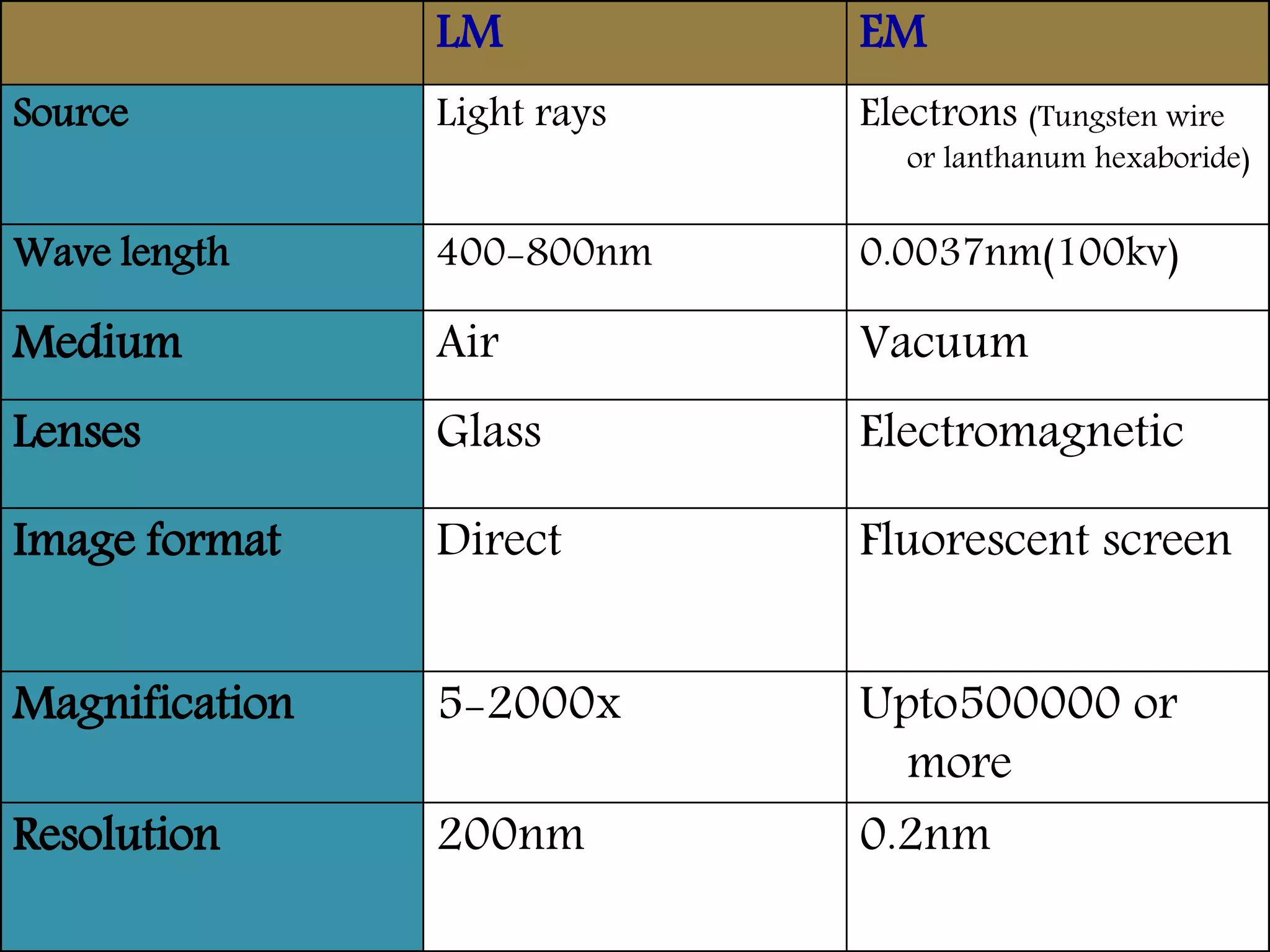
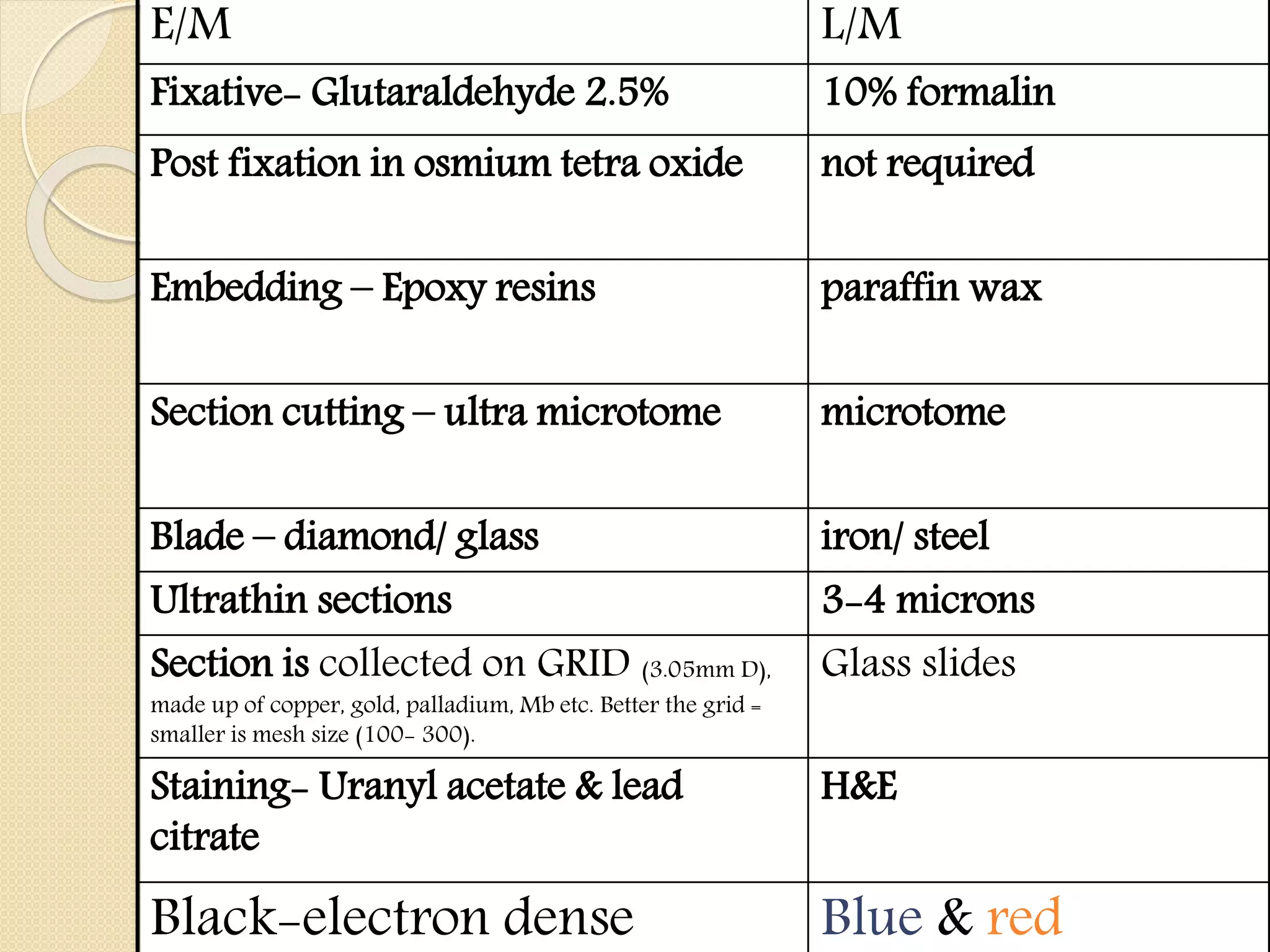
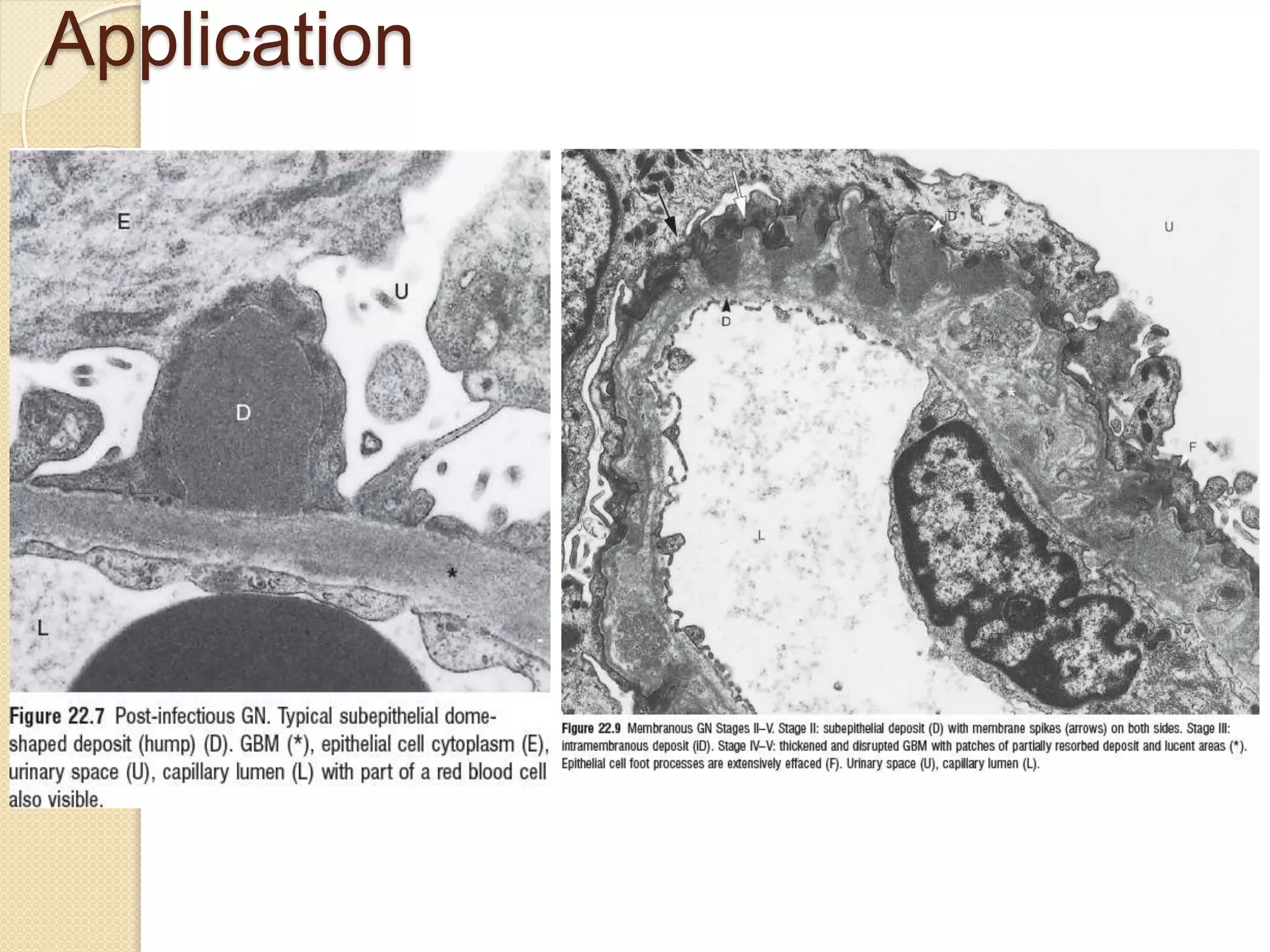
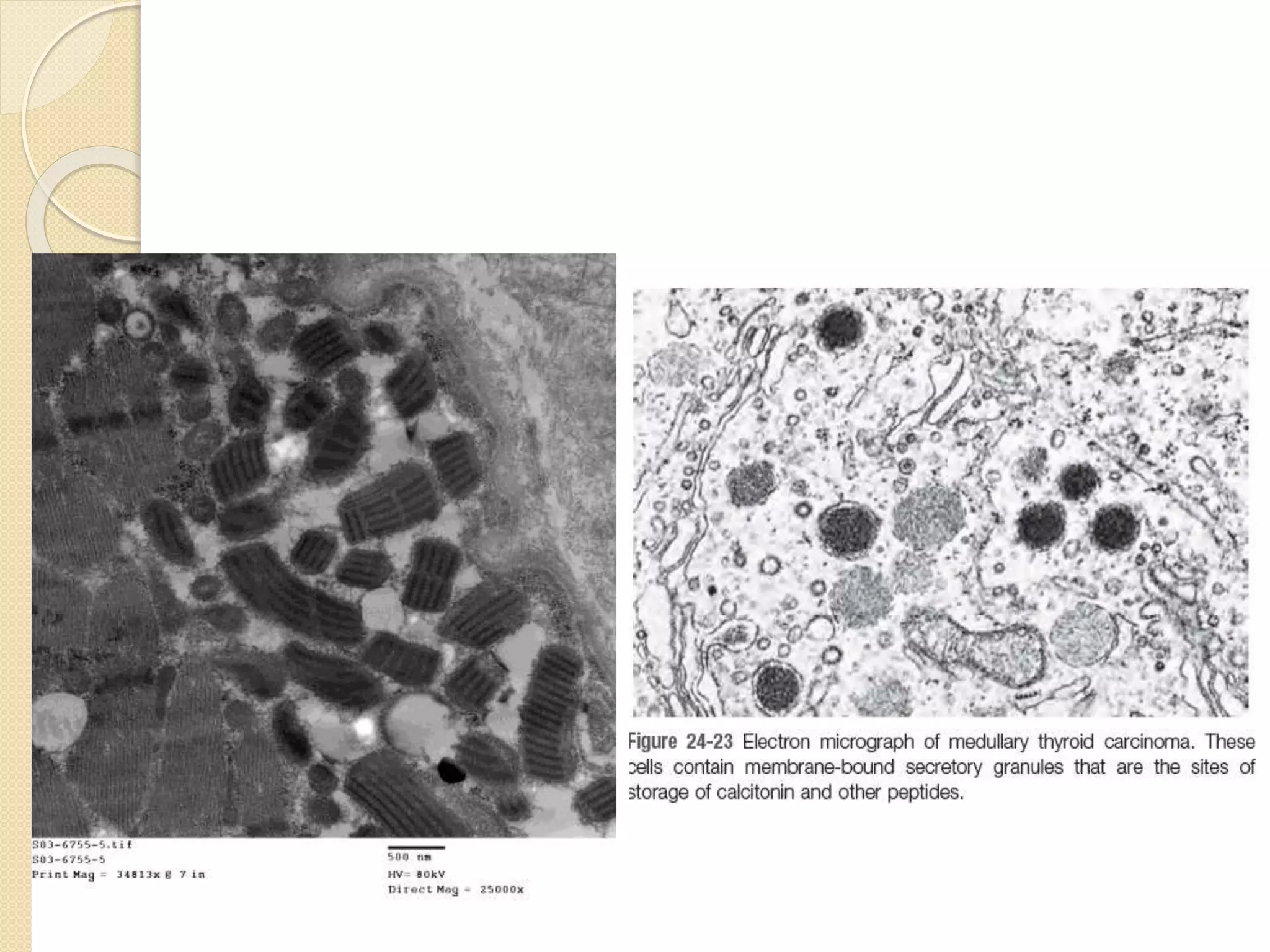
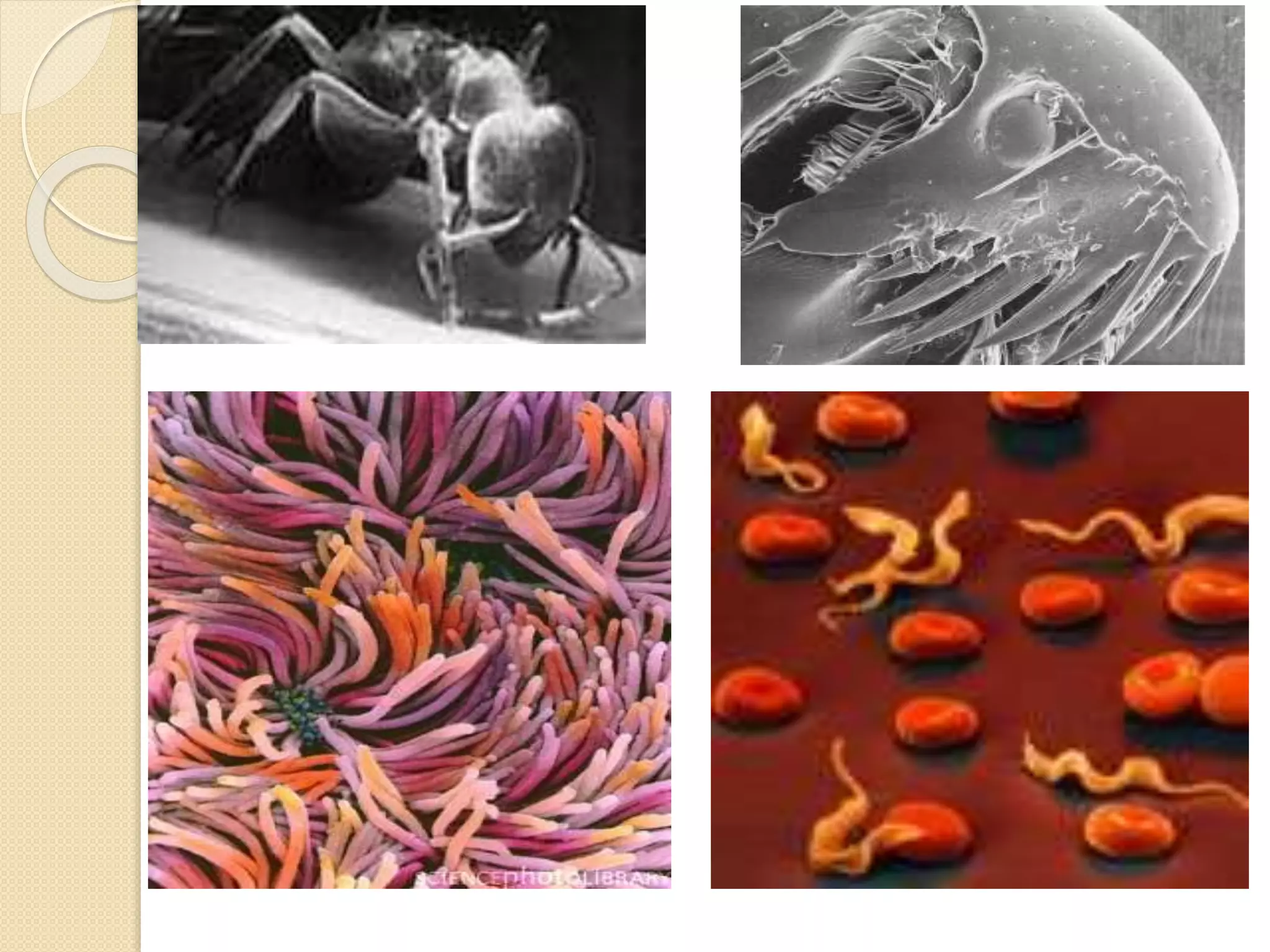
Electron microscopes use a beam of electrons instead of light to examine objects at a very fine scale, yielding morphological and topographical information. Max Knoll and Ernst Ruska invented the electron microscope in 1931, overcoming the resolution barrier of light microscopy. Improvements in electron optics, vacuum systems, and electron sources increased the resolution from 10 nm in the 1930s to 2 nm by 1944. There are two main types - transmission electron microscopes and scanning electron microscopes. Electron microscopes allow imaging of structures at the nanometer scale and provide information about structure and composition.