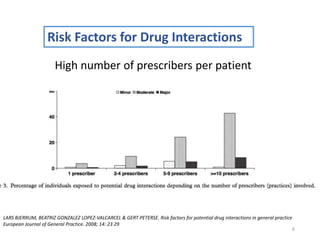
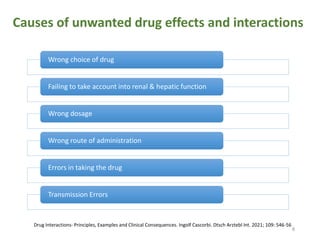
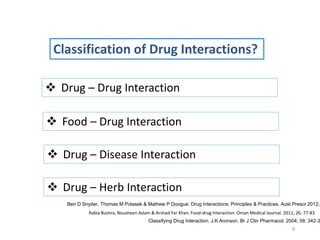
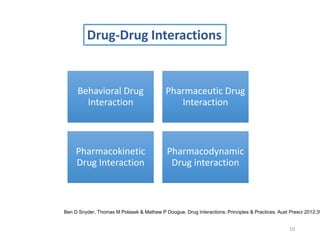
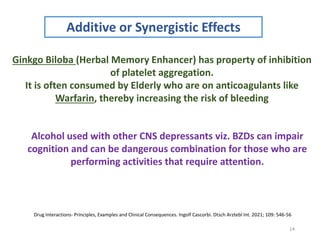
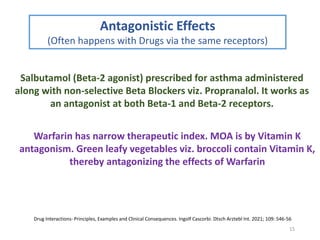
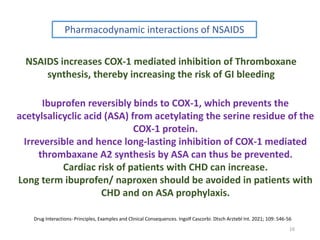
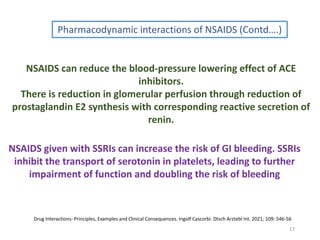
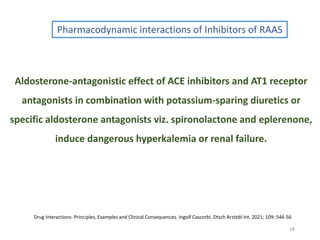
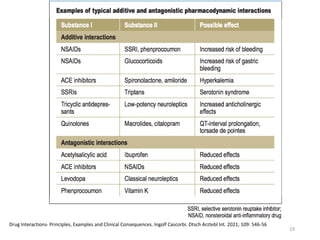
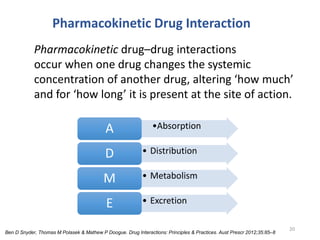
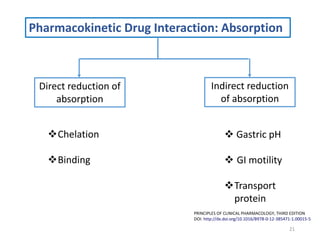
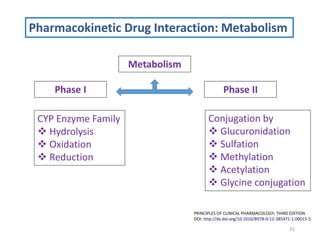
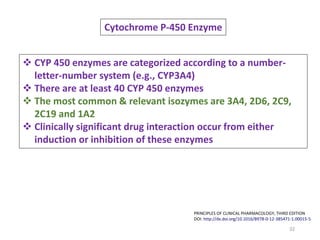
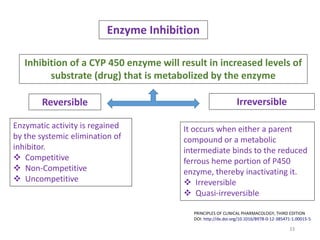
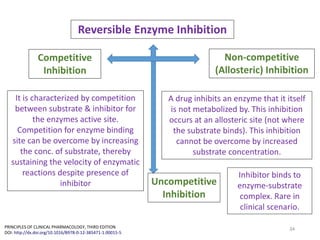
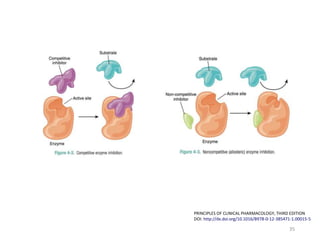
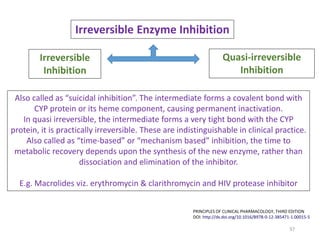
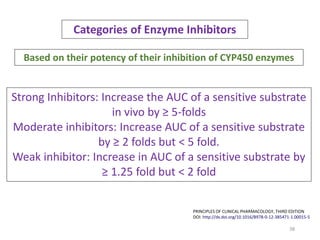
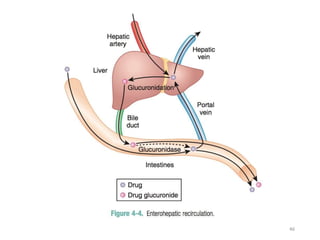
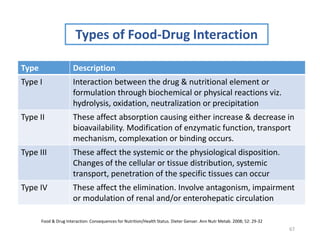
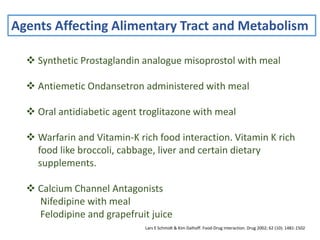
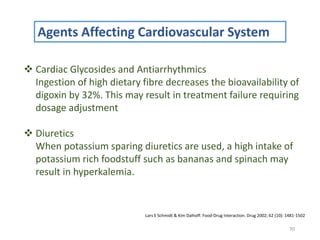
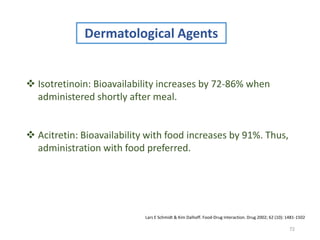
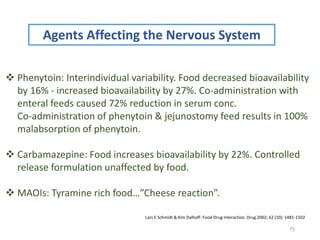
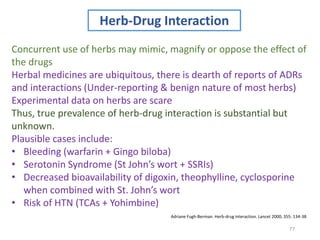
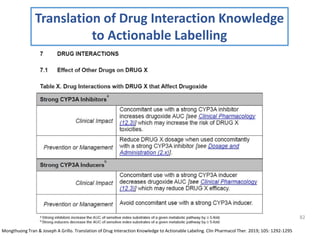
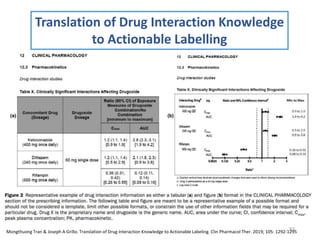
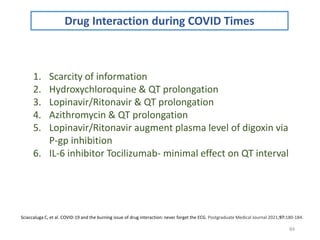
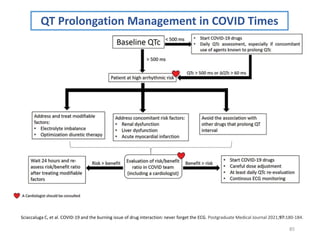
The document covers various aspects of drug interactions, including definitions, classifications (drug-drug, food-drug, drug-disease interactions), and their epidemiology, causes, and management strategies. It emphasizes the significant risk factors for drug interactions, such as polypharmacy and increasing age, and examines specific mechanisms like pharmacokinetic and pharmacodynamic interactions. It highlights the importance of awareness and management of these interactions to prevent adverse drug reactions and related morbidity.