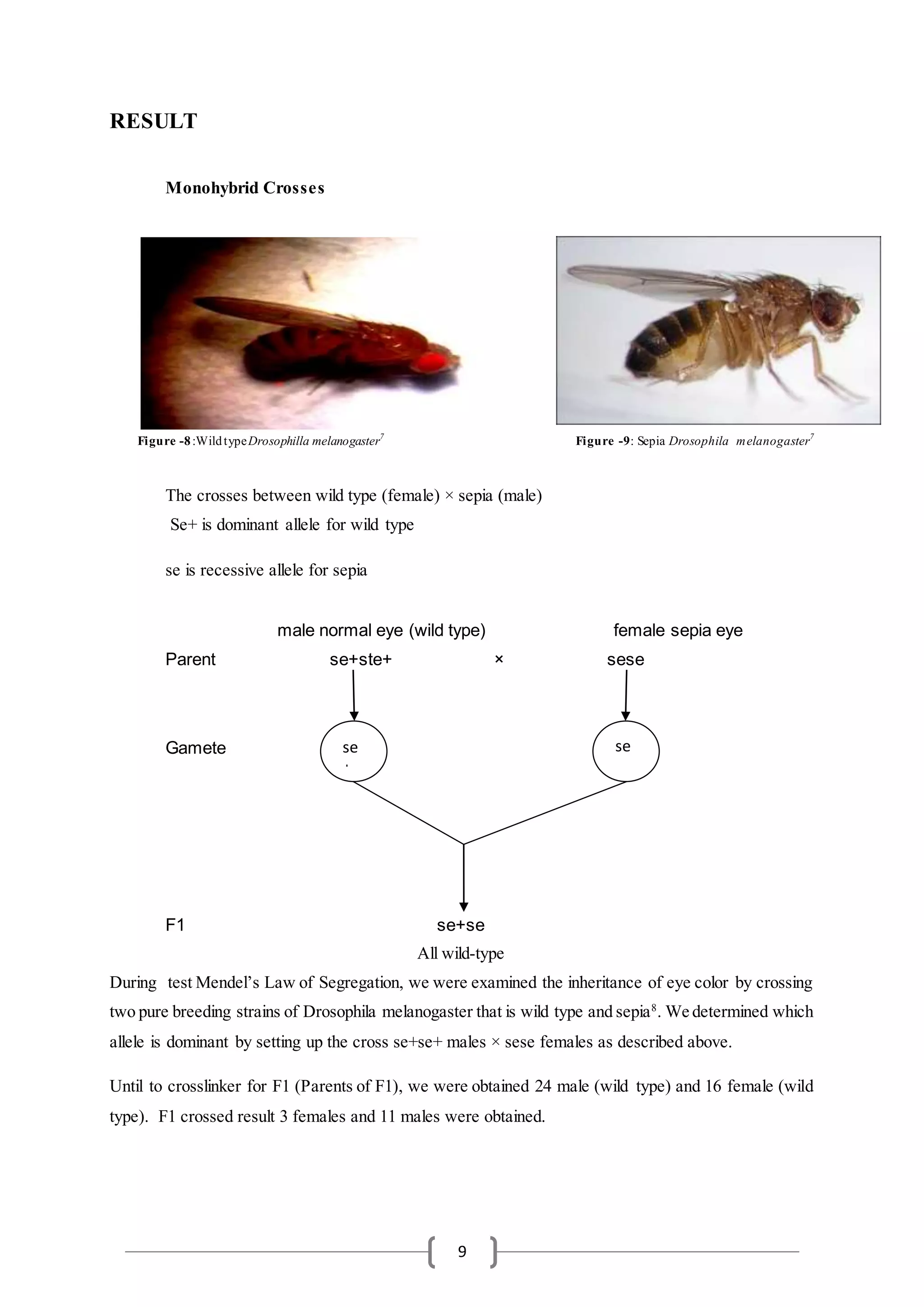
- The document describes an experiment involving Drosophila melanogaster flies to study genetic inheritance patterns.
- Wild type and sepia mutant flies were crossed, producing F1 offspring. The F1 offspring were then observed to determine phenotypic ratios.
- A total of 24 male and 16 female parental flies were obtained initially. After crosses and observations, 19 female and 20 male F1 offspring were observed, showing a 1:1 phenotypic ratio as expected based on Mendelian genetics.