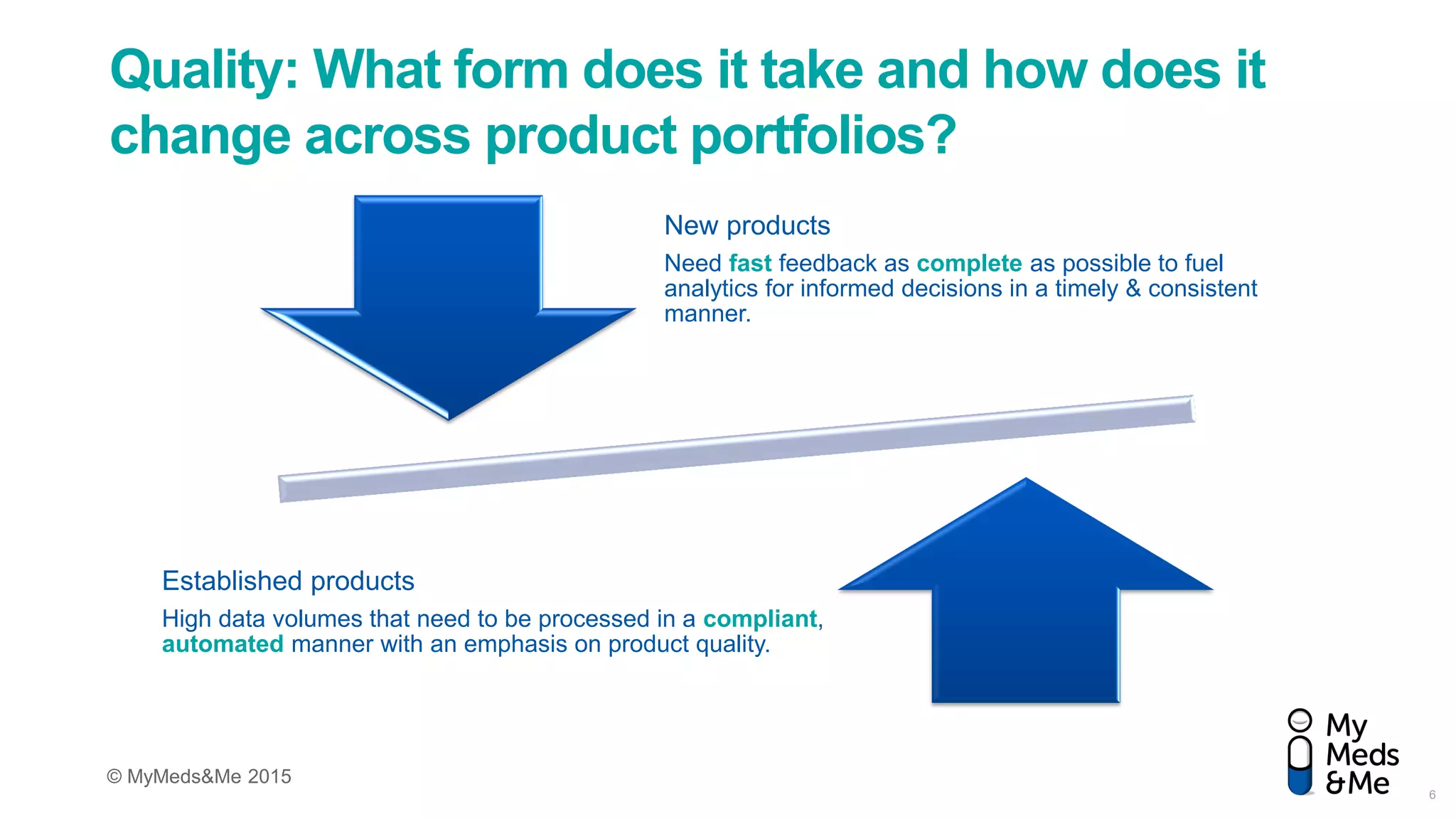
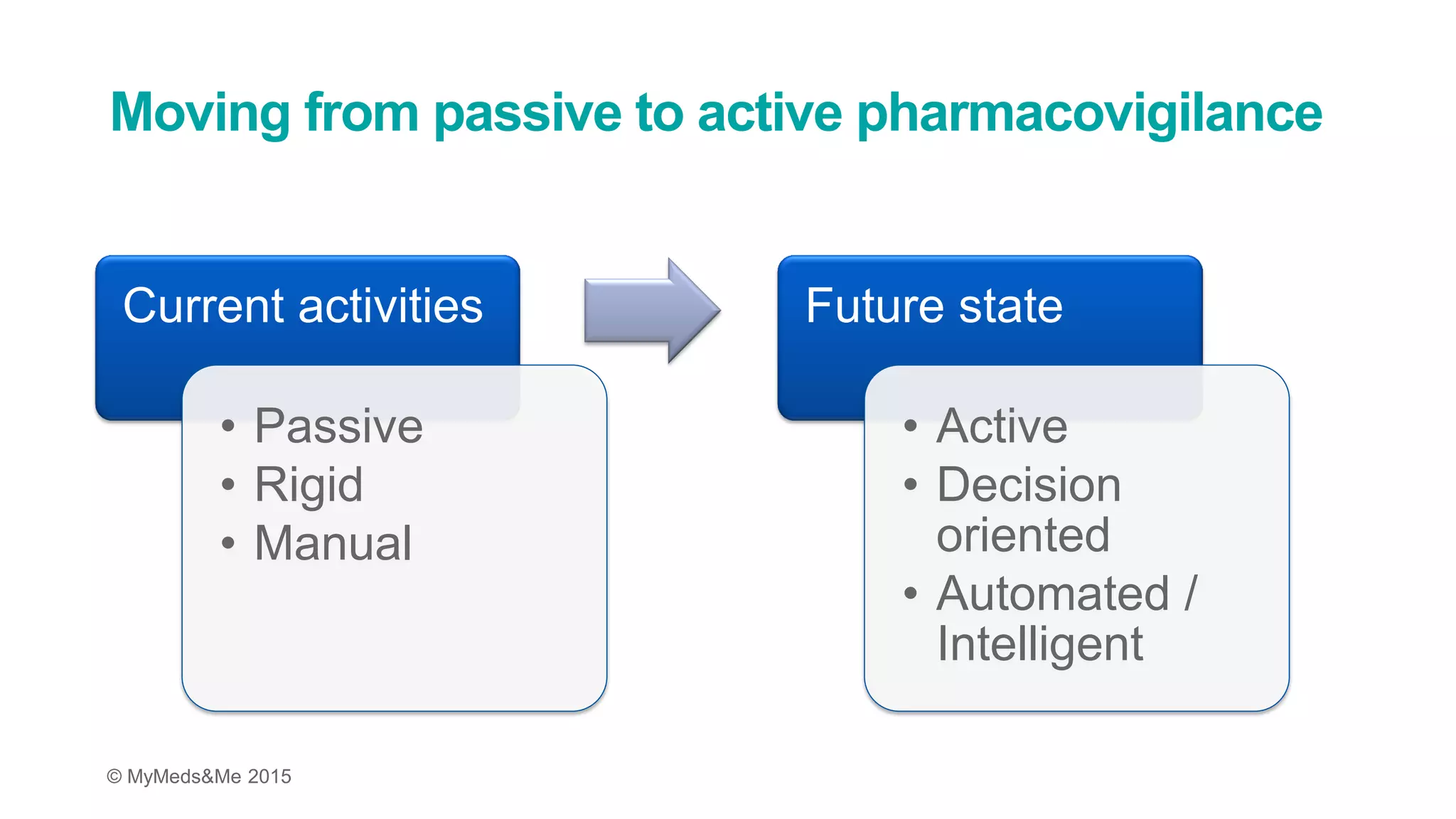
The document discusses the challenges in pharmacovigilance and risk management, highlighting a trend towards active pharmacovigilance using intelligent intake technology to improve decision-making. It emphasizes the need for quality data collection in the evolving landscape and the importance of meeting risk management commitments through innovative processes and automation. The implementation of this technology is presented as a scalable solution that can adapt to organizational changes and reduce costs.