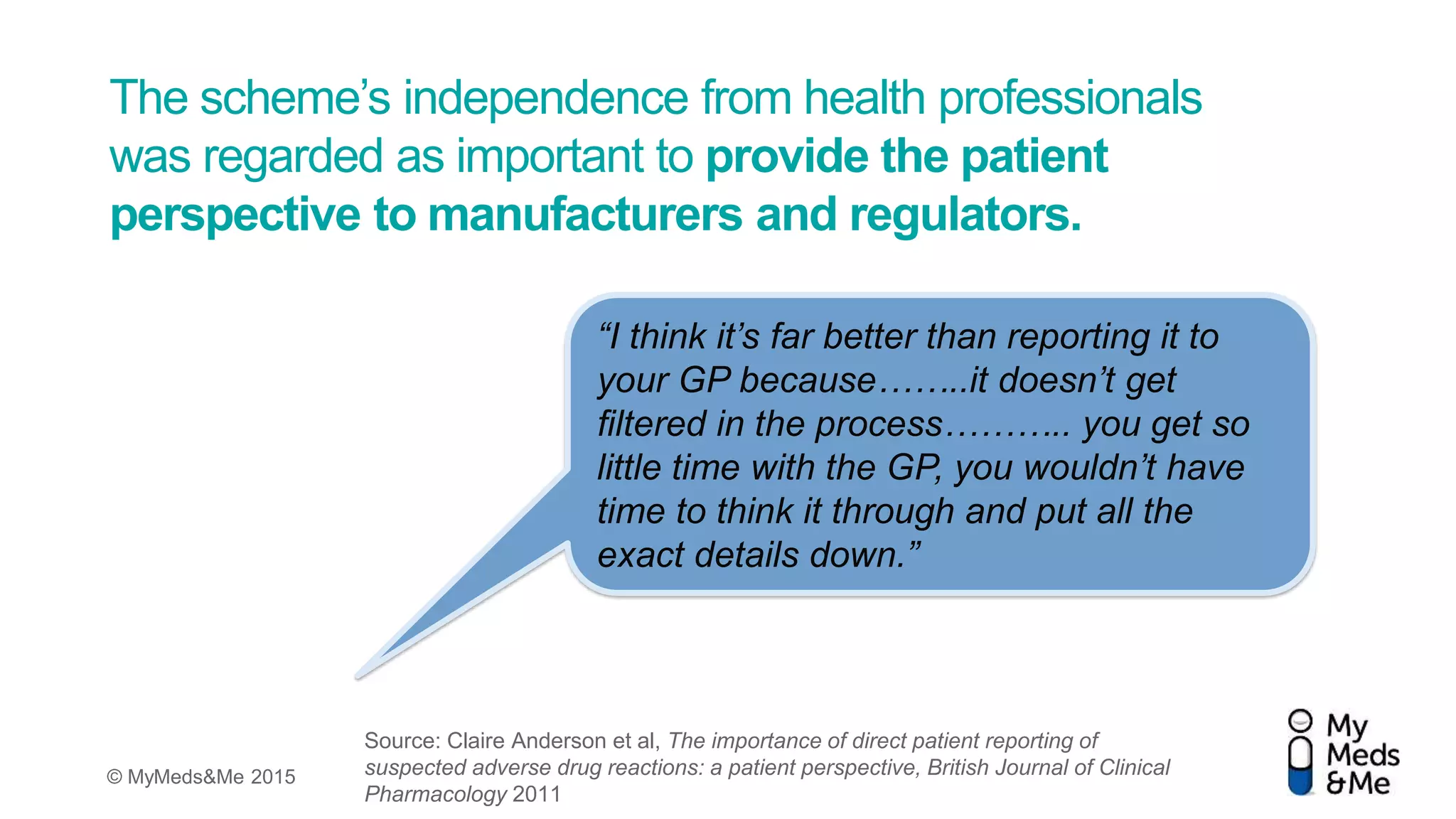
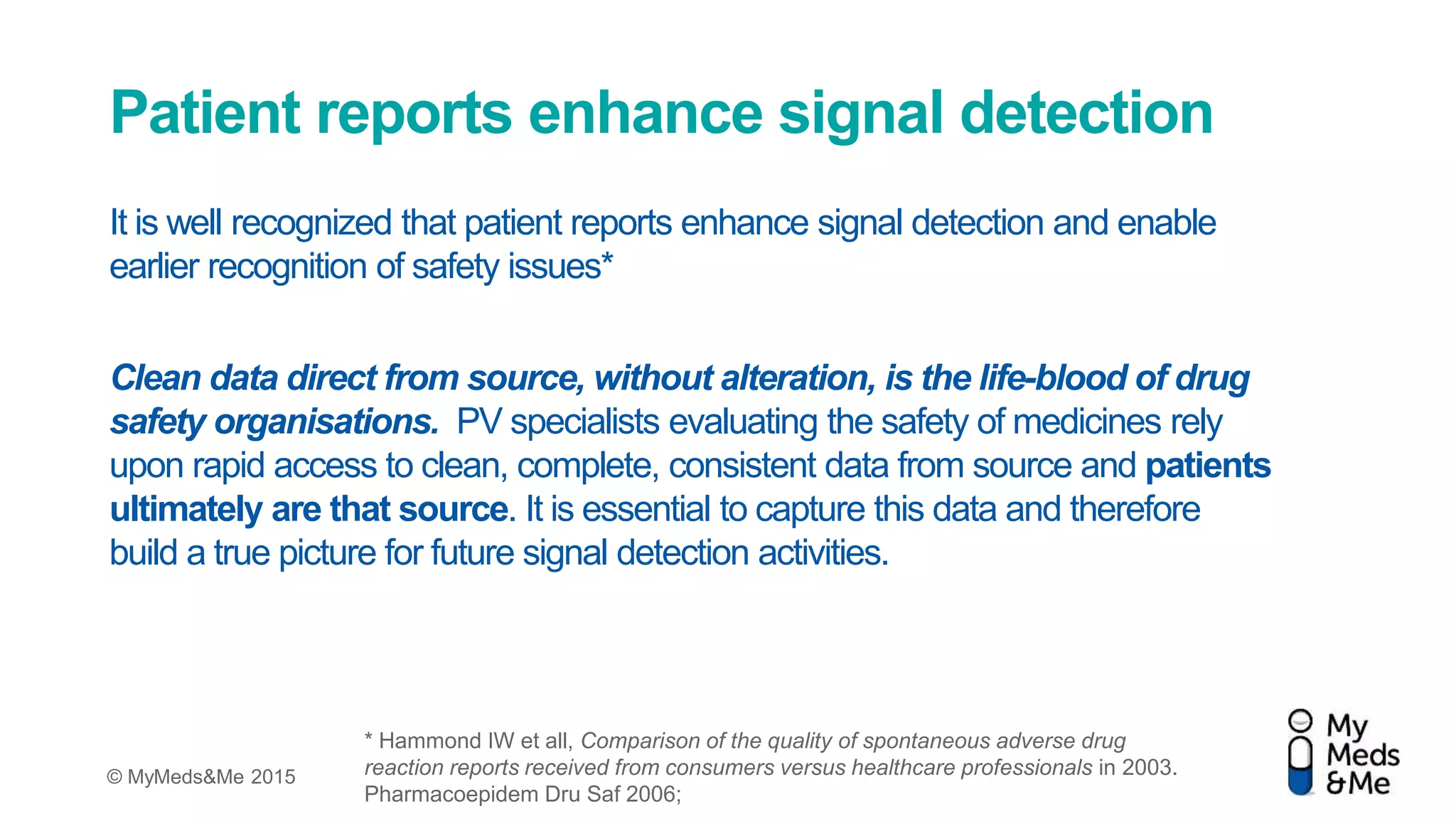
The document presents a debate on the diminishing role of healthcare professionals (HCPs) in yellow card reporting for adverse drug reactions, emphasizing that despite increased reports of adverse reactions globally, HCP reporting remains low in the UK. It highlights significant under-reporting by HCPs compared to patient reports and advocates for improving patient awareness and simplifying reporting processes through electronic means. Ultimately, it suggests that direct patient reporting of adverse drug reactions is vital for pharmacovigilance and can enhance data quality and safety monitoring.






![© MyMeds&Me 2015
Let’s put this in context
How many prescriptions are issued annually in the UK?
1 Billion*
How many OTC medicine packs are sold per year?
Again, 1 Billion
And finally, what% of people experience an adverse event whentaking a medicine?
On average 10%
So based on these numbers, whatis the level of under-reporting?
Being conservative somewhere around 100 fold!! Being conservative somewhere around 100 fold!! Only
between<1% - 6%** of suspectedADRs experienced by patients are reported.
Think back to the 75,000 hospital admissions due toADRs……. in just one year
* HSCIC, Prescriptions dispensed in the Community, Statistics for England – 2002-2012 [NS], July 30, 2013
** Hazell et al, Under-Reporting of Adverse Drug Reactions,– May 2006, Drug Safety, Vol. 29, Issue 5
** Y. Moride et al, Under-reporting of adverse drug reactions in general practice – Oct 2003](https://image.slidesharecdn.com/debateround-upslidesandrewrutfinal-150331154455-conversion-gate01/75/Debating-the-Future-of-Spontaneous-Reporting-Dr-Andrew-Rut-CEO-and-Founder-MyMeds-Me-presents-at-MHRA-Scientific-Conference-20-March-2015-7-2048.jpg)