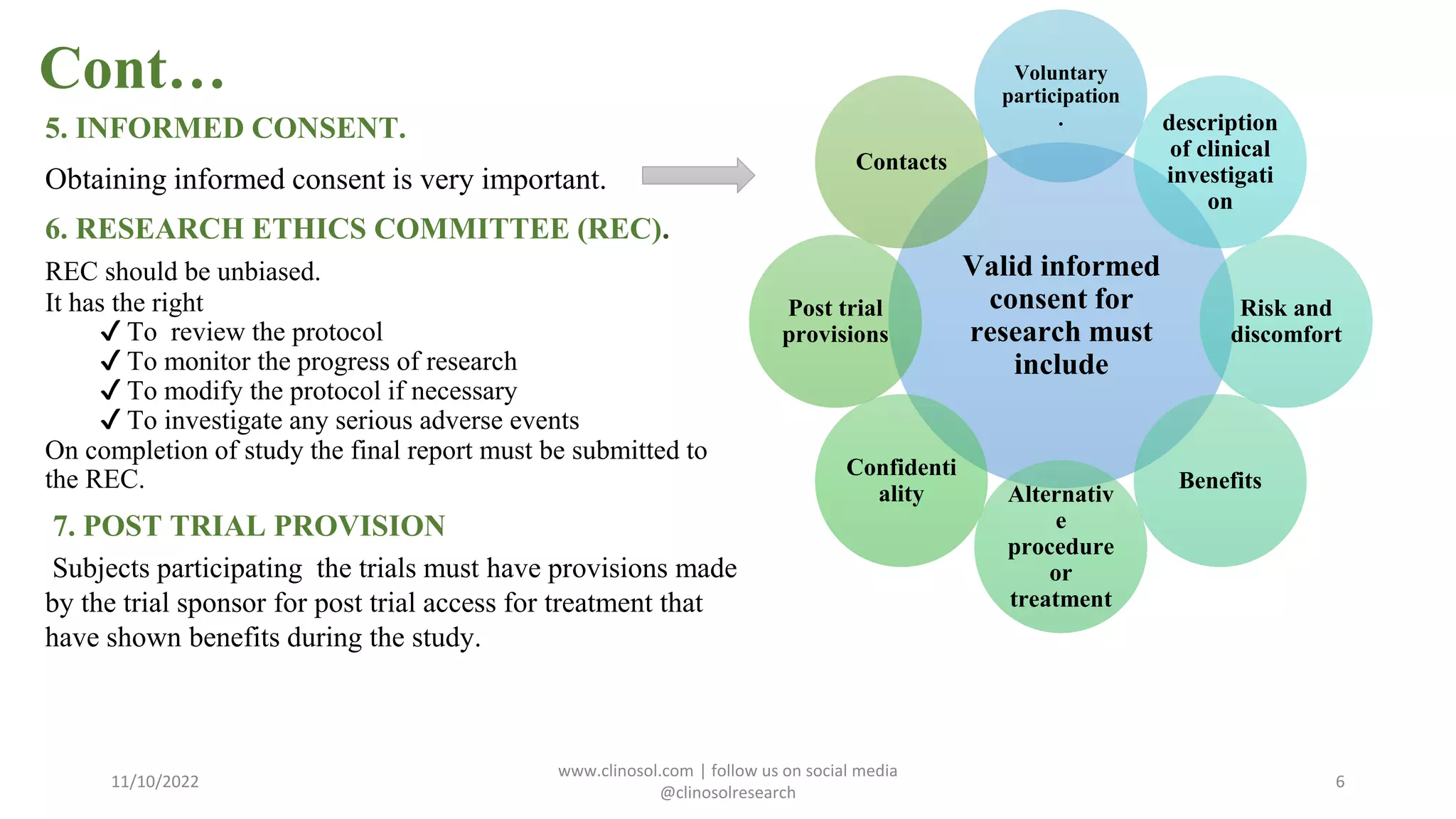
The Declaration of Helsinki outlines ethical principles for medical research involving human subjects, emphasizing the importance of informed consent, risk-benefit analysis, and the protection of vulnerable groups. It has undergone multiple amendments since its initial adoption in 1964, reflecting evolving ethical standards in clinical research. Researchers are required to adhere to these principles and local regulations when conducting studies involving human participants.