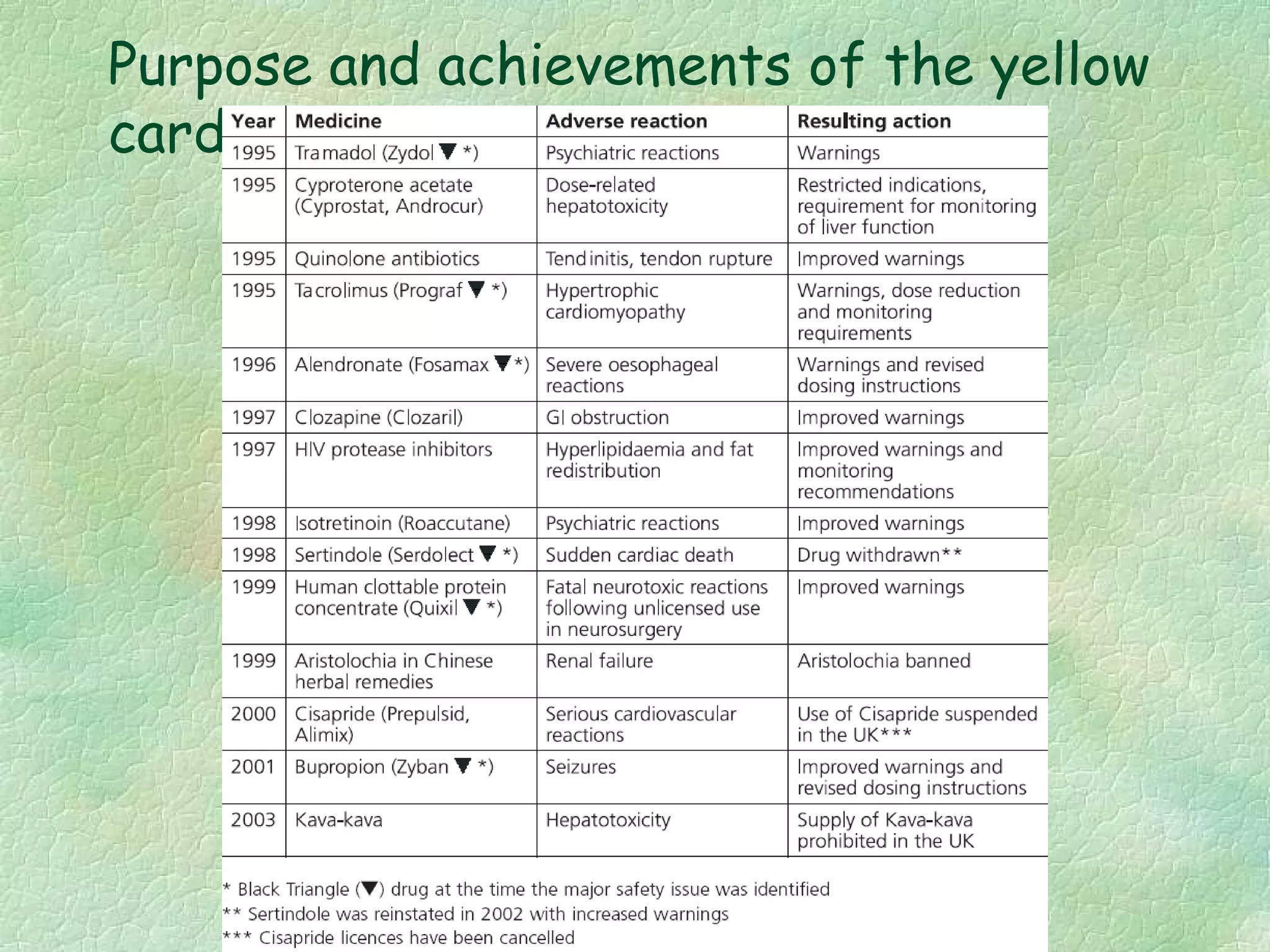
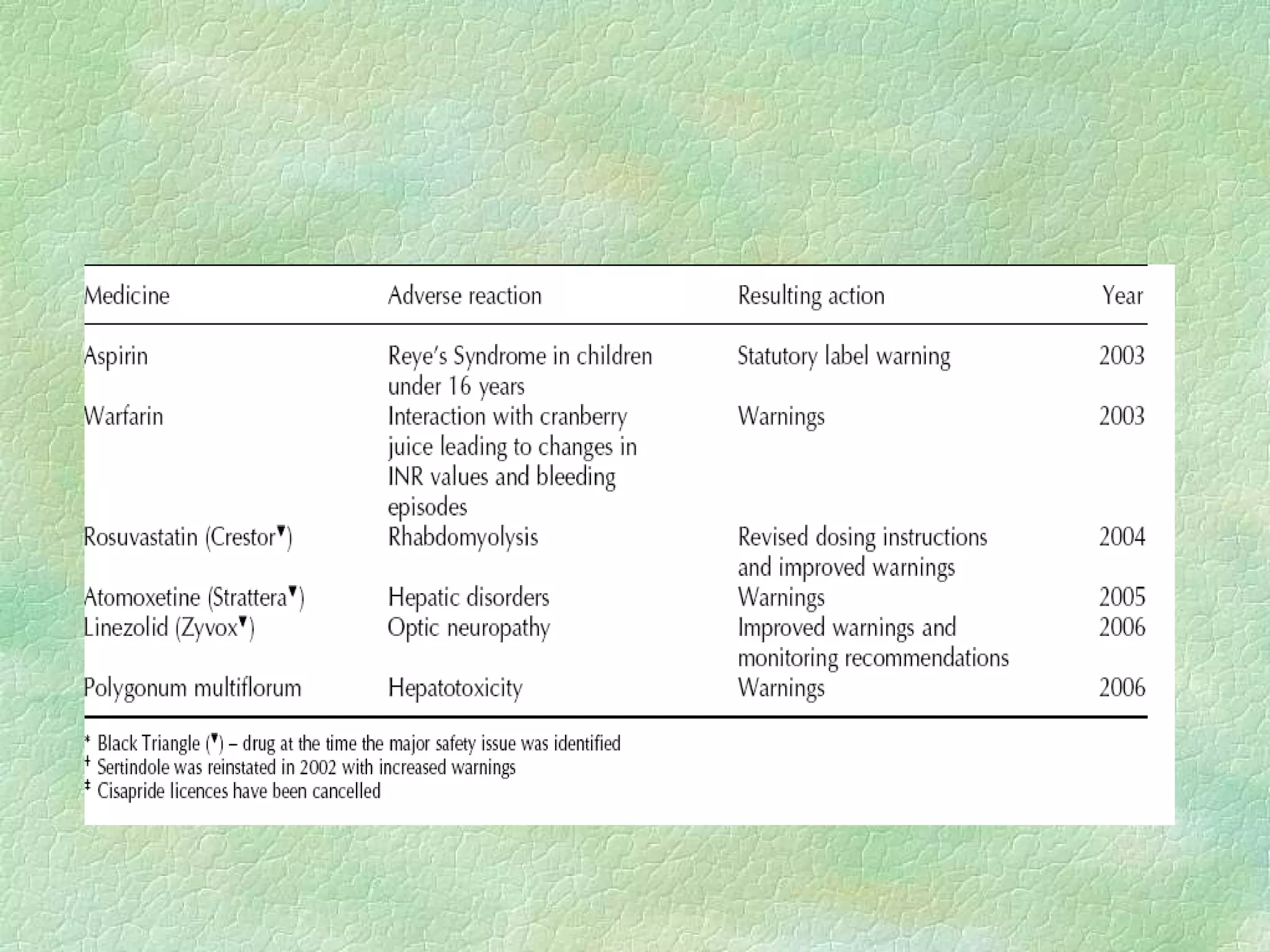
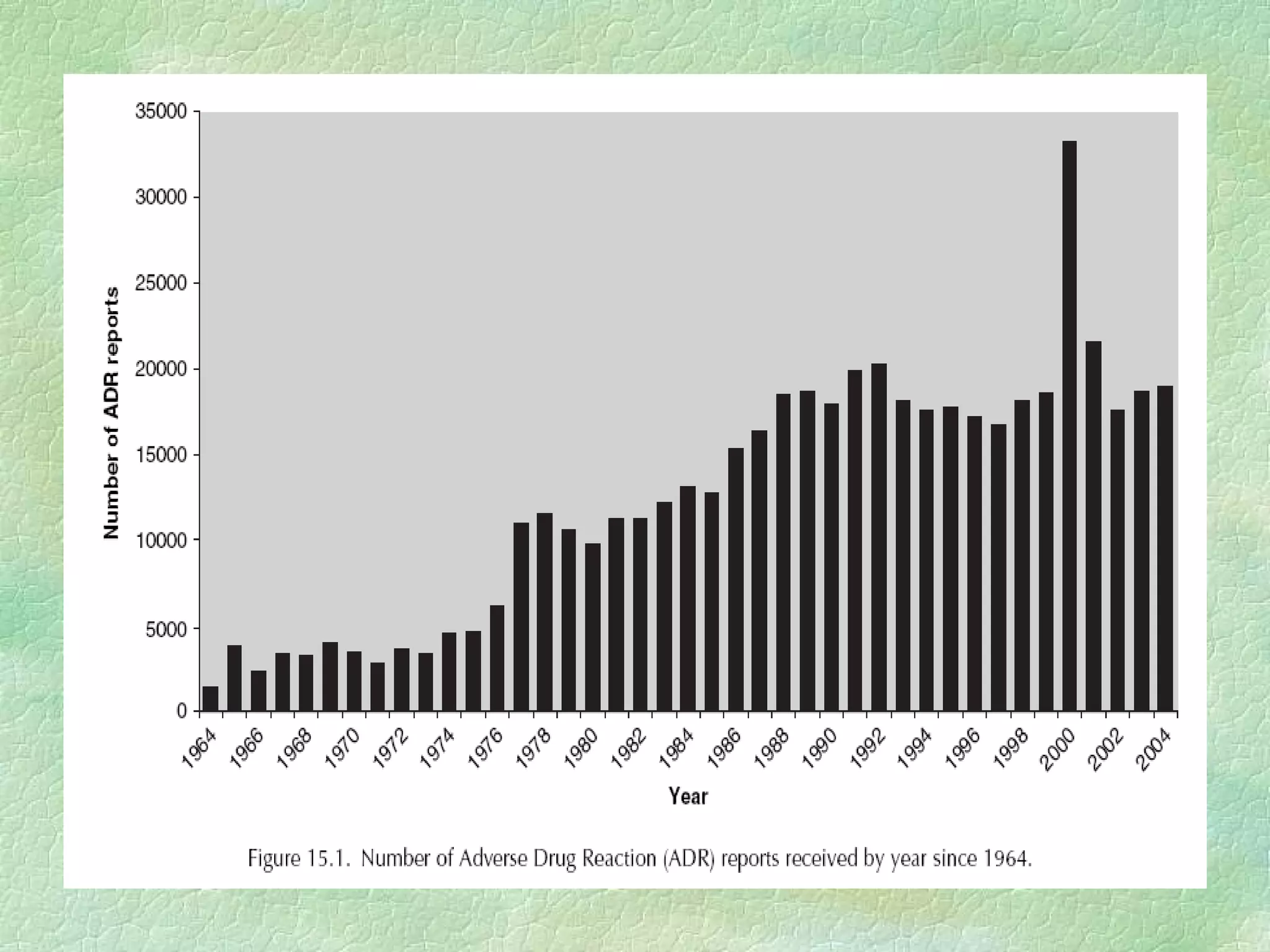
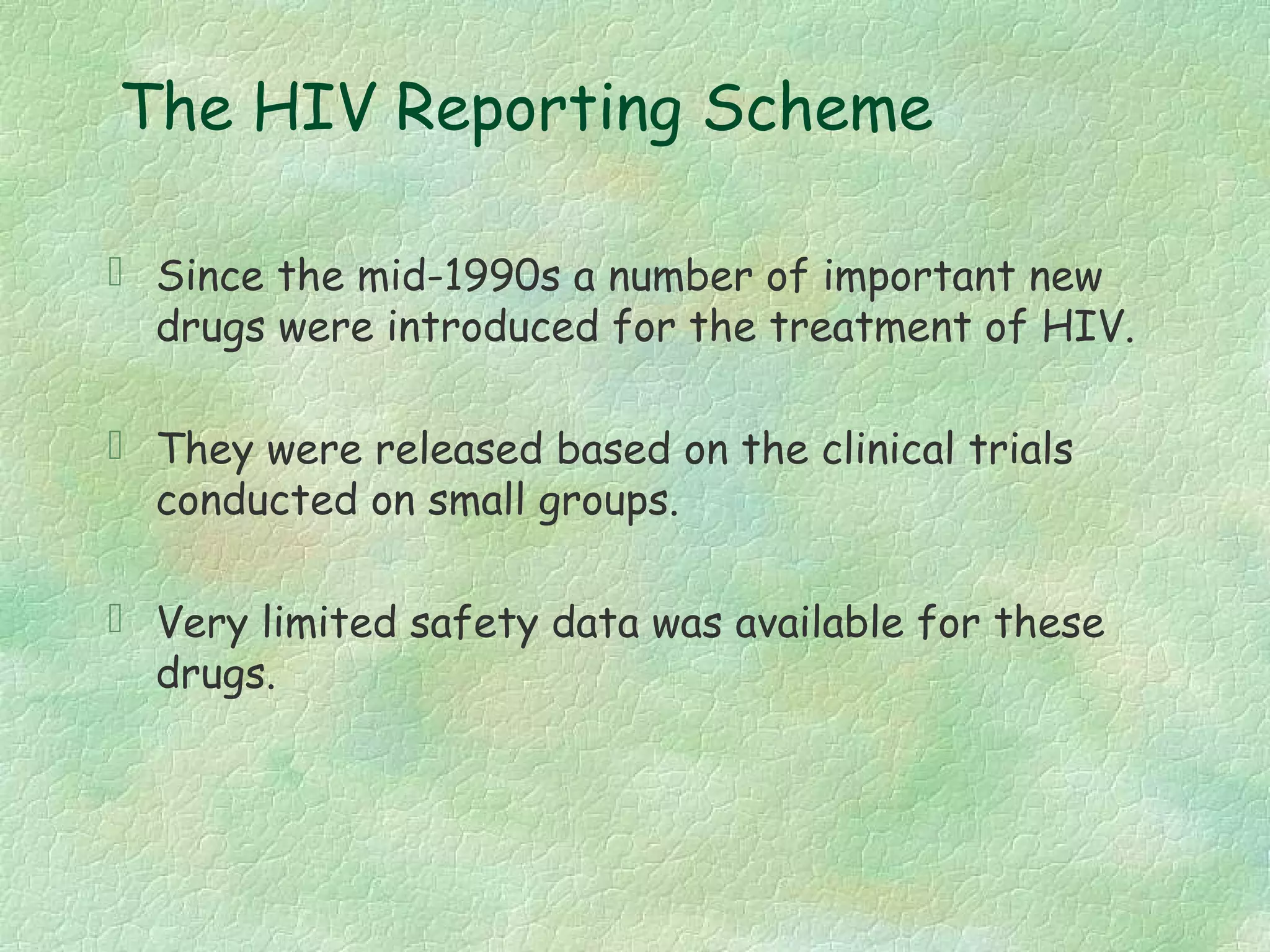
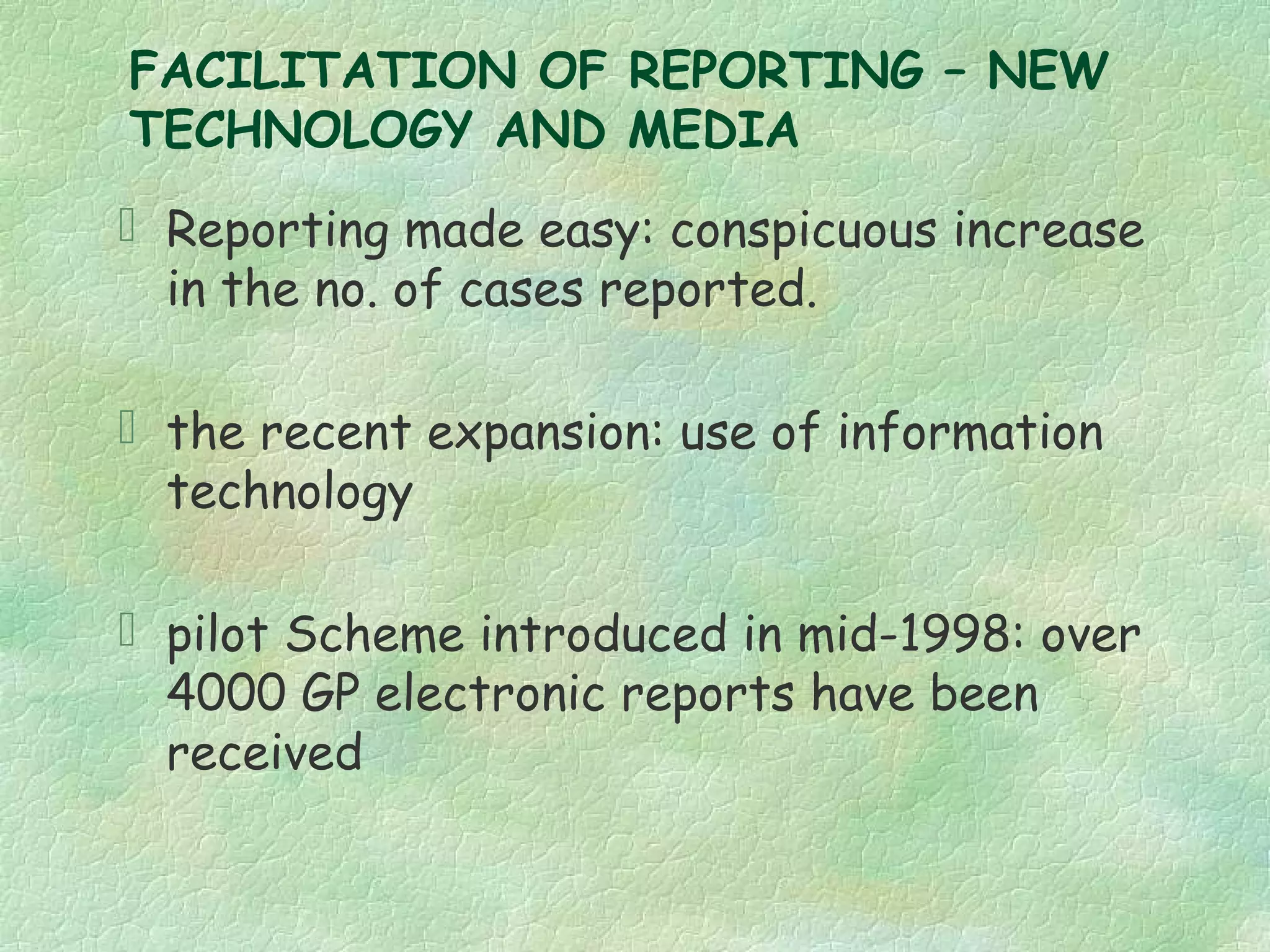
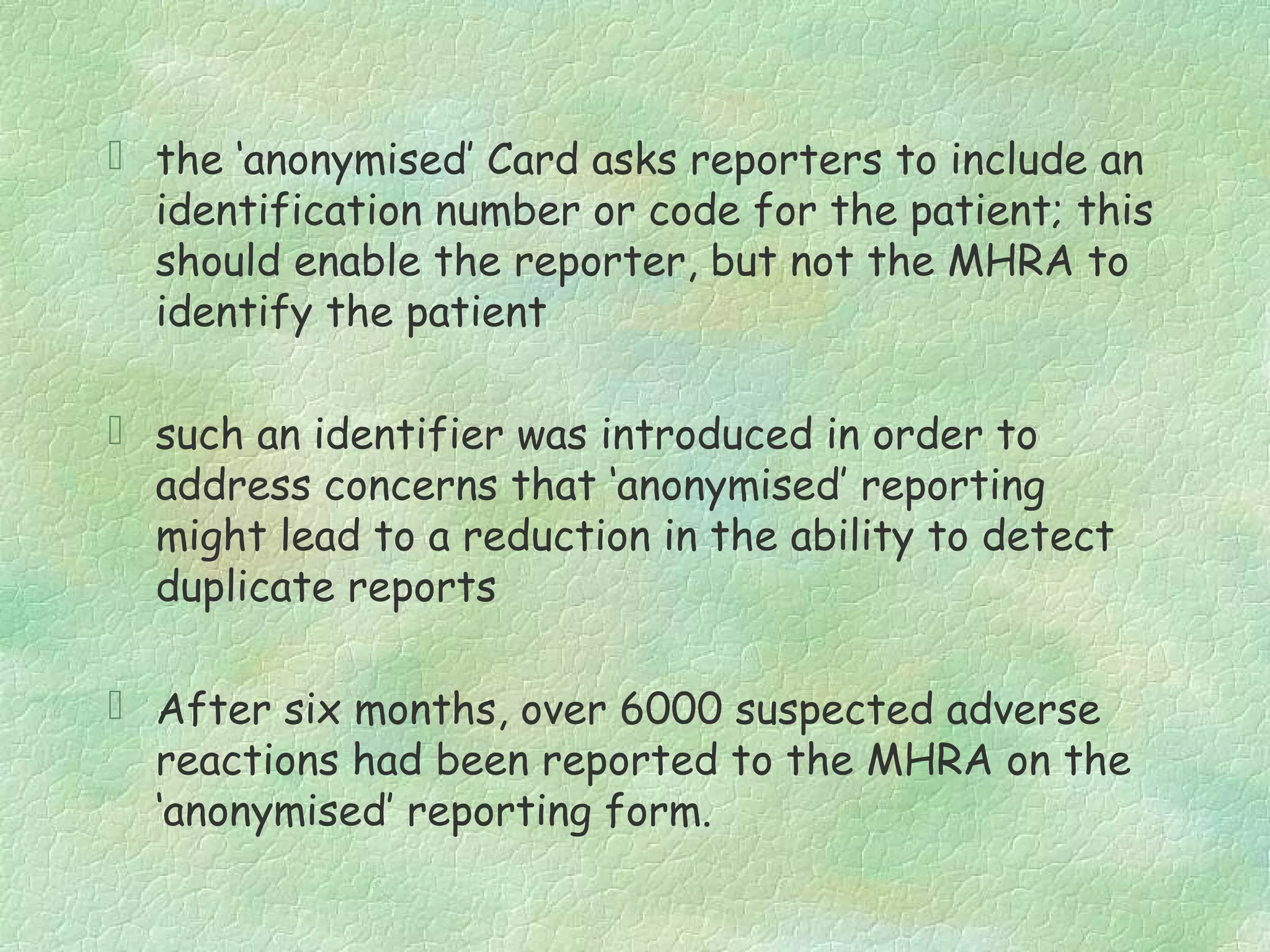
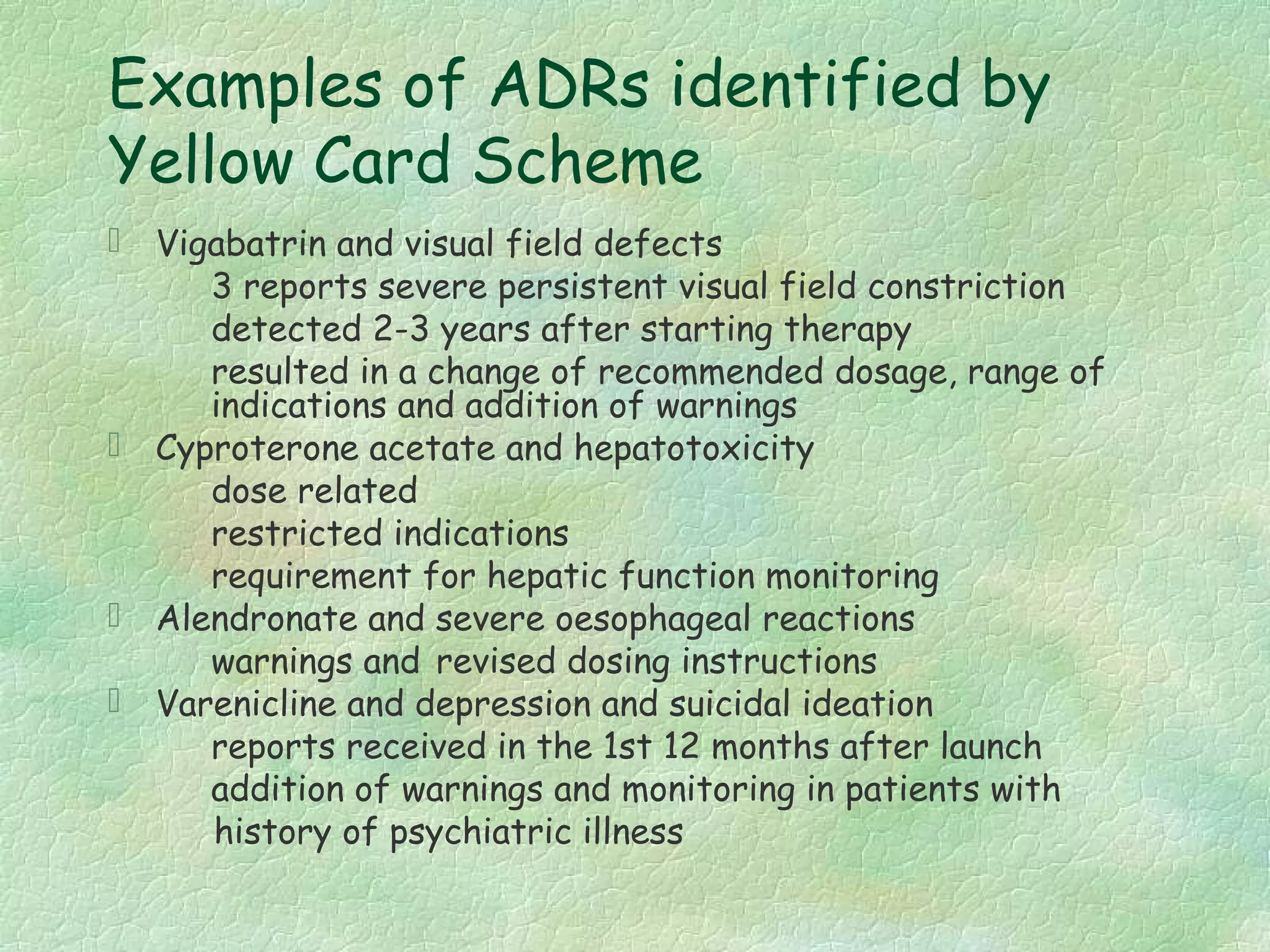
The spontaneous reporting system is a passive surveillance system where health professionals voluntarily report adverse drug reactions directly to regulatory authorities or pharmaceutical companies. It involves 3 main processes: 1) data acquisition from reported cases, 2) assessment of individual reports and pooled data, and 3) interpretation of signals based on the available data. Initiatives have been taken to widen reporting bases to include pharmacists and nurses. Special focus has been given to reporting reactions related to HIV drugs, pediatric drugs, and herbal products to improve surveillance in underreported areas.