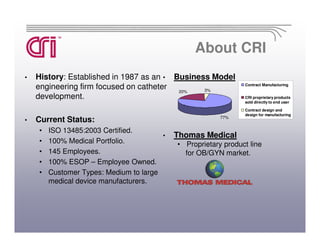
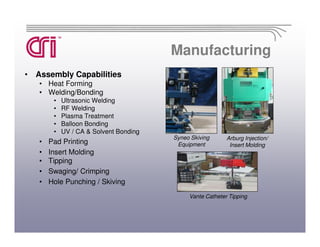
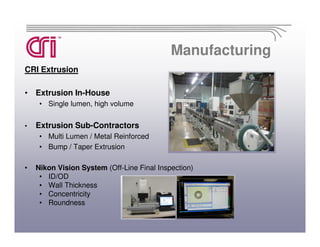
Catheter Research Inc. is a leading medical device manufacturer specializing in catheter design, medical tubing, and OBGYN disposables. It has 145 employees and is ISO 13485 certified. CRI provides contract manufacturing services for medium and large medical device companies as well as produces its own proprietary OBGYN product line under the Thomas Medical brand.