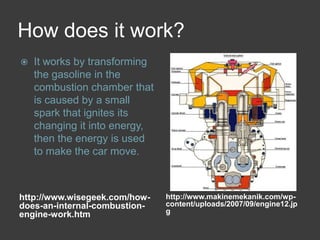
This document discusses combustion reactions and provides examples of combustion. A combustion reaction is when oxygen combines with a substance, such as wood, producing water and carbon dioxide. It requires the fire triangle elements of heat, fuel, and oxygen. The cooktop stove and car engines are examples that rely on combustion. A combustion engine works by sparking gasoline in the combustion chamber, transforming it into energy that powers the car. NASCAR engines are specially designed for high-speed combustion without turbocharging. Nitrous oxide increases combustion by providing extra oxygen.