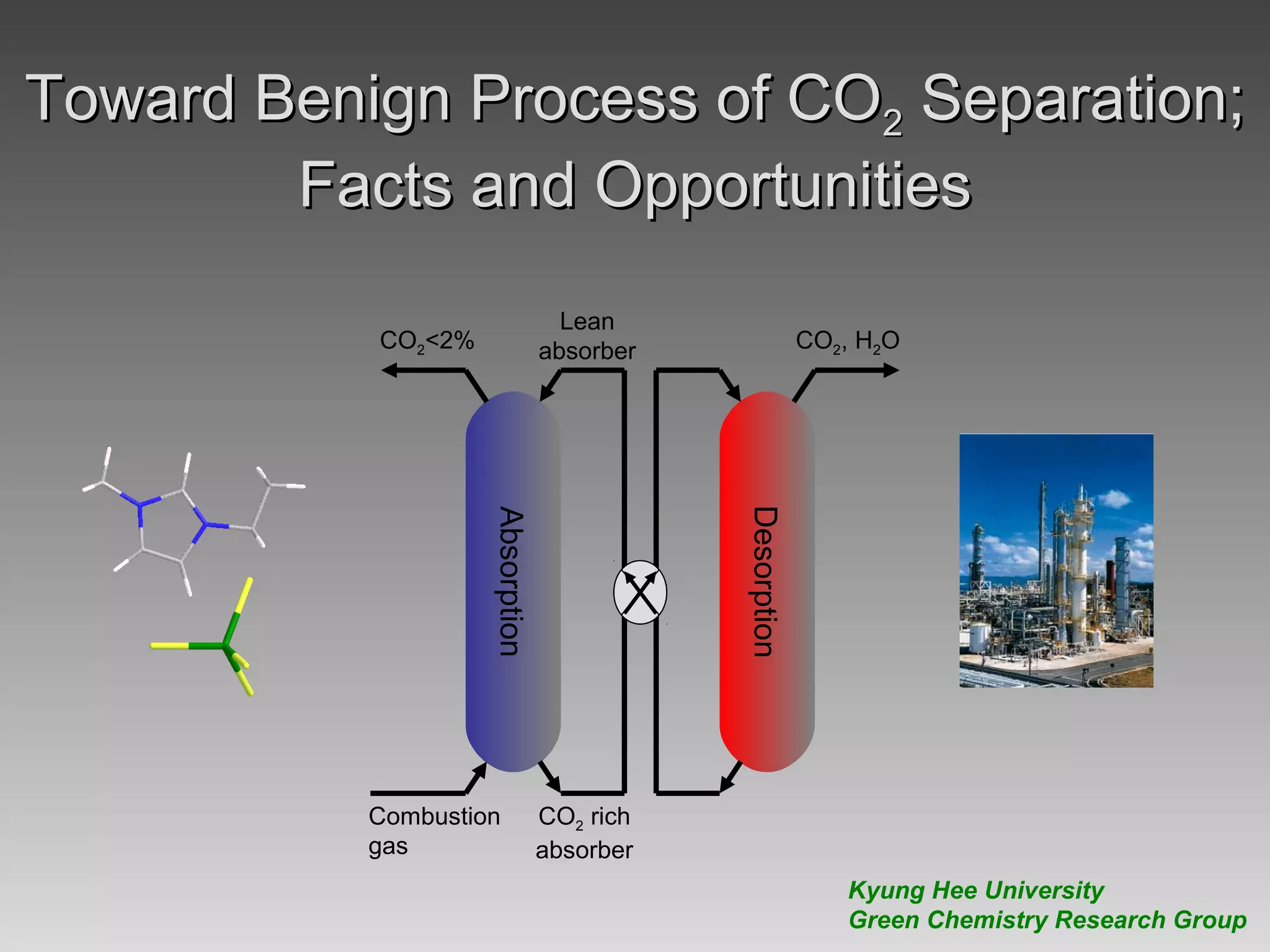
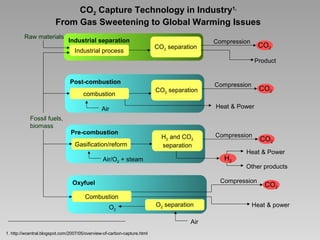
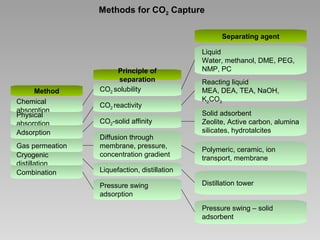
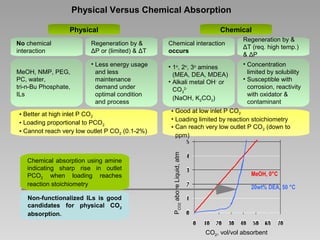
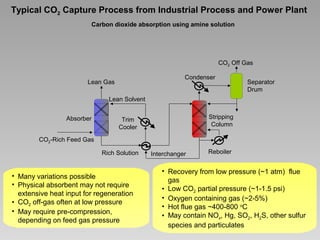
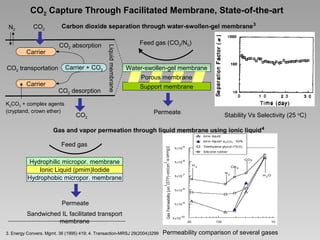
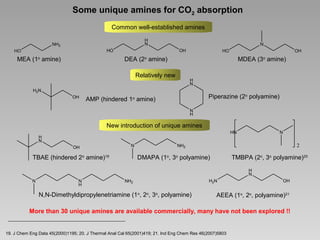
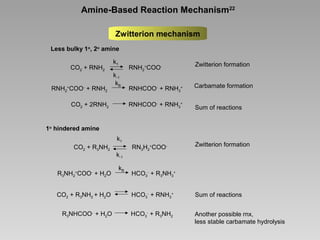
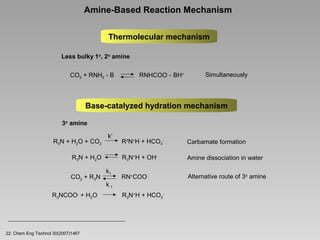
This document discusses various methods for capturing carbon dioxide (CO2) from industrial processes and power plant flue gases. It describes both established and developing absorption-based techniques using liquid solvents such as amines, ionic liquids, and hyperbranched polymers. While amine scrubbing is a mature process, opportunities exist to improve solvent capacity and reduce regeneration energy needs through new solvent formulations and process designs. Developing technologies like facilitated transport membranes and task-specific ionic liquids also aim to enhance CO2 capture efficiency. Fundamental research on reaction mechanisms and new candidate materials continues to inform the design of more effective and economical CO2 capture systems.


![Anion and Cation Effects on the Solubility of CO2
70
O
[bmim][PF6]
60
[bmim][BF4]
F3C
[bmim][Tf2N]
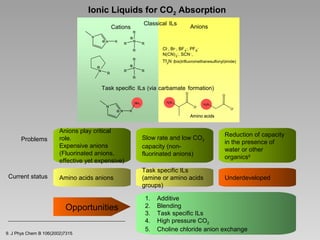
50
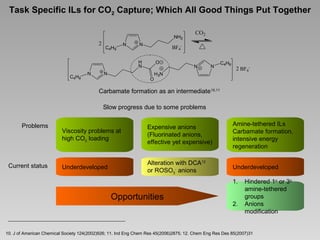
[MeBu3N][Tf2N]
40
S
S
O
[MeBuPyrr][Tf2N]
N
O
Anion effect
Cation effect
10
0
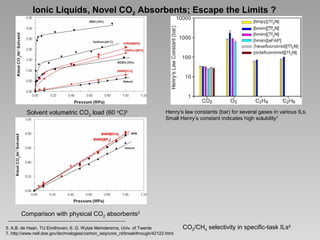
Henry’s law constants (bar) for CO2 in various ILs
CF3
F
F
F
P
F
F
F
determining the
CO2 solubility3;
The anions
30
20
O
Fluorinated anions are excellent but
costly, sometimes not
environmentally friendly.
Some strategies in ILs for CO2 absorption8
Creating more free
volume (introducing
ether and long,
branched alkyl chain
on the cation
8. Journal of Physical Chemistry B 109 (2005) 6366
Incorporating with CO2phylic functional groups
(carbonyl, sulfonyl,
phosphate, amine
groups)
Controlling the viscosity
(using dicyanamide
anions, trialkylsulfonium
cation)](https://image.slidesharecdn.com/co2separation-greenchem-proposal-131025084018-phpapp02/85/CO2-Separation-A-Proposal-10-320.jpg)
![Poly(ionic liquid)s; Creative Effort13,14,15
*
*
*
n
*
*
*
n
*
*
n
n
x
*
*
1-x
O
x
*
*
1-x
O
O
O
O
O
O
BF4N
N
R
R
R
P[VBTMA][BF4]
BF4
-
N
O
O
n
N
N
R
n
O
P[VBBI][BF4]
P[MABI]BF4
P[VBTMA][BF4]-g-PEG
O
P[MATMA][BF4]-g-PEG
Brittle materials
Current status
O
N
R
Problems
BF4N
R
P[MATMA][BF4]
BF4-
BF4-
N
BF4-
R
R
O
O
Thermodynamics,
kinetics and
mechanism
Low efficiency
Grafting polymers
In progress, limited
results available
Underdeveloped
1.
Opportunities
13. Chem Commun (2005)3325; 14. Ind Eng Chem Res 46(2007)5397; 15. J Membrane Sci 281(2006)130
2.
ILs grafted onto
selective polymers
Specific
membrane](https://image.slidesharecdn.com/co2separation-greenchem-proposal-131025084018-phpapp02/85/CO2-Separation-A-Proposal-13-320.jpg)
![Total Equilibrium
Pressure (KPa)
Total Equilibrium Pressure (KPa)
Total Pressure equilibrium Vs CO2 capacity (30 mins capacity (30 mins run)
Total Pressure equilibrium Vs CO2 run)
80. 0
80.00
80. 0
Total Equilibrium
Pressure (KPa)
Total equilibrium pressure (KPa)
100.00
60. 0
60.00
60. 0
40. 0
40.00
0.00 5.00
5.00
0.00
10.00
10.00
5.00
10.00
0. 0
15.00
15.00
15.00
mole of CO2/2kg of absorbent
mole of CO / kg of absorbent
60.0
40. 040.0
20. 0
20.00
80.0
20. 020.0
0. 0
0. 0
20.00
0.0
20.00
20.00
0. 4
0.0 0.0
mole of CO2/ kg of absorbent
1mL MDEA + + 4mL H2O
1mL MDEA 4mL H2O
5mL 4.76%wt 2- methylimidazole/ H2O
5mL 4. 76%wt 2- methylimidazole/ H2O
5mL 4.76%wt imidazole/ H2O
5mL 4. 76%wt imidazole/ H2O
5mL 4.76%wt guanidine carbonate/ H2O
5mL 4. 76%wt guanidine carbonate/ H2O
5mL 4.76%wt DMEA/ H2O
5mL 4. 76%wt DMEA/ H2O
5mL 4.76%wt DAP
5mL 4. 76%wt DAP
2mL MDEA +2mL MDEA + 3mL H2O
3mL H2O
5mL 23.08%wt K2CO3/ H2O
5mL 23. 08%wt K2CO3/ H2O
5mL 23.08%wt K2CO3/ H2O K2CO3/ H2O
5mL 23.08%wt
5mL 4.76%wt Naglycine/ H2O
5mL 4.76%wt Naglycine/ H2O
5mL 4.76%wt Naglycine/ H2O
5mL 4.76%wt 1,2- dimethylimidazole/ H2O
5mL 4.76%wt 1,2- dimethylimidazole/ H2O 5mL 4.76%wt Naglycine/ H2O
5mL 4.76%wt MEA/ H2O MEA/ H2O
5mL 4.76%wt
5mL 4.76%wt MEA/ H2O
5mL 4.76%wt MEA/ H2O
5mL 4.76%wt DMEA/ H2O DMEA/ H2O
5mL 4.76%wt
1mL DMEA + + 4mL H2O
1mL DMEA 4mL H2O
4mL 4.76%wt DAP + 1 mL DMEA 1 mL DMEA
4mL 4.76%wt DAP +
1mL
5mL
5mL
5mL
1mL
5mL
2. 0
2.4 2.4
MDEA +1mL MDEA + 4mL H2O
4mL H2O
4.76%wt 2- methylimidazole/ H2O
5mL 4. 76%wt 2- methylimidazol
4.76%wt 1,2- dimethylimidazole/ H2O
5mL 4. 76%wt 1,2- dimethylimid
9.09%wt guan-09%wt guan- car/ H2O
5mL 9. car/ H2O
DMEA +1mL DMEA + 4mL H2O
4mL H2O
4.76%wt DAP 76%wt DAP
5mL 4.
Capacity (mol of CO2/mol absorbent) of various amines
CO
Loading (mol of CO2/kgSolubility (Validation of Experiments)
absorbent) of various amines
2
16
160
[emim]etOSO3
120
CO2 mole fraction (x1000)
140
PCO2 equilibrium (KPa)
1.6
0.0. 8
4
0.8
1.2.0
6
0.4
0.8 1.2 1.2 1.2 1.6
2.0
a (molmol absorbent)
a (mol CO2(mol2/CO2absorbent)
a / CO mol / mol absorbent)
Jones et al; J Chem Eng Data4(1959)85
Shen et al; J Chem Eng Data 37(1992)96
Song et al; J Chem Eng Data 41(1996)497
This work
100
80
60
40
20
0
[emim]etOSO3 + 7.0% w/w ZnBr2
[emim]etOSO3 + 7.0% w/w sugar
12
[bmim]BF4
8
4
0
0. 4
0.5
0. 6
Capacity (mol CO2/ mol of MEA)
0. 7
Experimental Validation of CO2 solubility test (15.3%wt. MEA)
30
50
70
90
110
Pequilibrium (KPa)
Effect of additive on the CO2 absorption capacity of simple ILs](https://image.slidesharecdn.com/co2separation-greenchem-proposal-131025084018-phpapp02/85/CO2-Separation-A-Proposal-21-320.jpg)