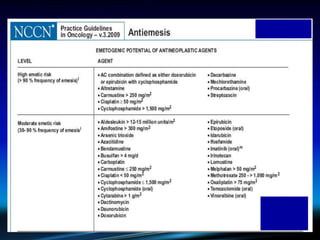
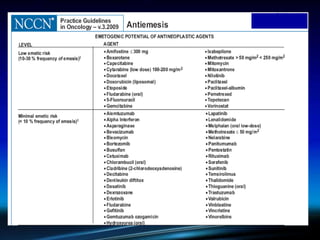
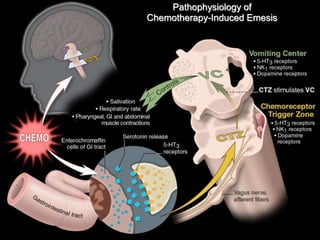
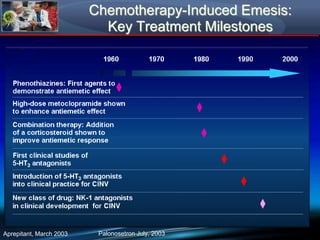
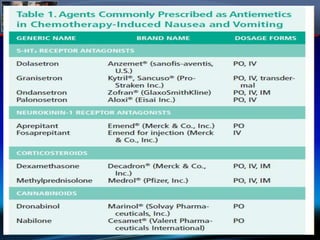
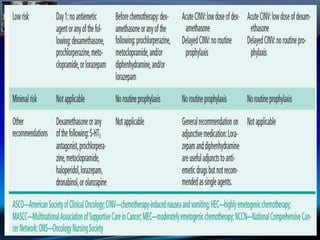
Chemotherapy-induced nausea and vomiting (CINV) poses varying emetogenic risks, categorized as high, moderate, low, and minimal, with specific patient risk factors influencing susceptibility. Treatment options include corticosteroids, dopamine antagonists, serotonin (5-HT3) antagonists, and NK-1 receptor antagonists, with palonosetron showing superior efficacy and prolonged action. Despite advancements, challenges remain in improving detection and management of CINV, particularly in the delayed phase.