Embed presentation
Download to read offline



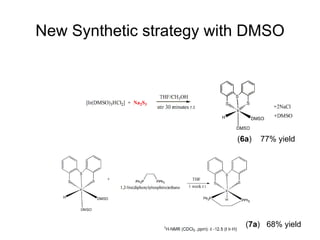



The document discusses the design of catalytic systems for selectively hydrogenating carbon-oxygen double bonds in furan derivatives. It describes the synthesis of pre-catalysts using cis-dichloride and unsaturation. New synthetic strategies were developed using dimethyl sulfoxide and 1,5-cyclooctadiene, yielding various products in high yields. Several new complexes were synthesized and characterized using NMR spectroscopy. Novel synthetic methods allowed tuning of ancillary ligands to control steric and electronic effects at the metal center.






