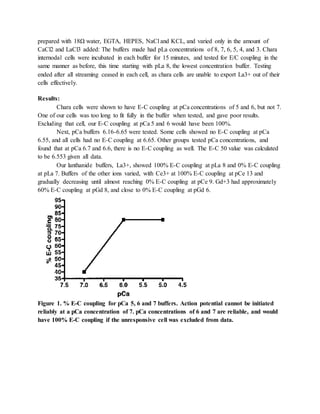
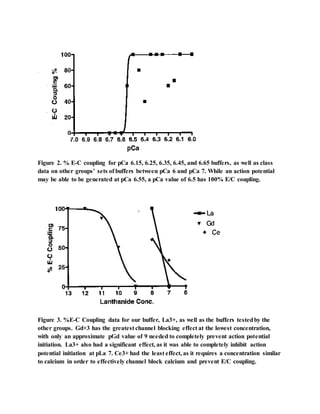
This document summarizes a study that investigated the effect of calcium ion concentration on action potential initiation in Chara internodal cells. The study found that a calcium concentration of at least pCa 6.5 is needed to reliably generate an action potential. Lanthanide ions like La3+ can inhibit action potential generation by blocking calcium channel entry, with pLa 7 being sufficient to prevent action potential initiation. Future experiments could compare the calcium concentration needed for mechanical versus electrical stimulation to generate an action potential.