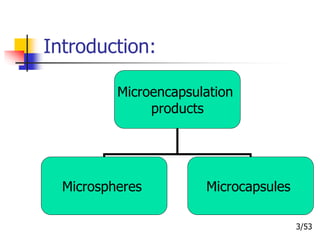
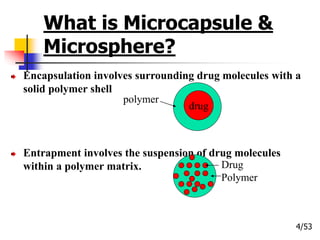
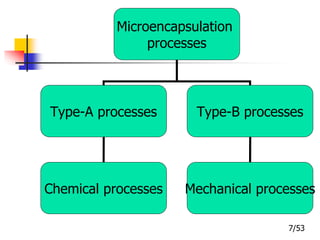
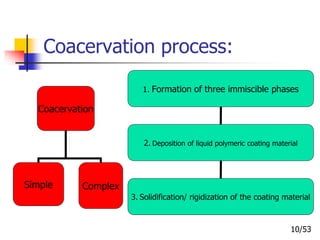
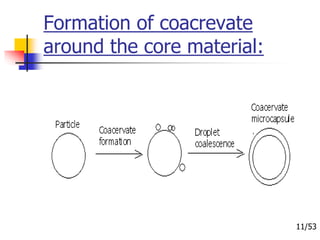
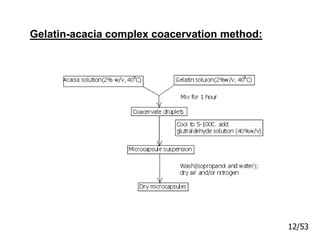
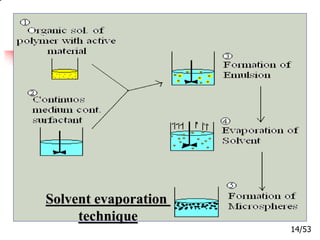
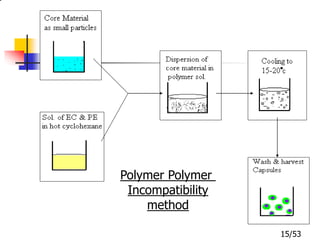
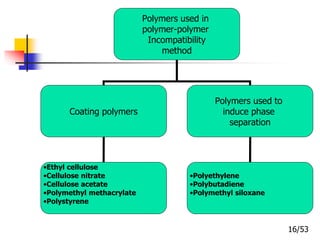
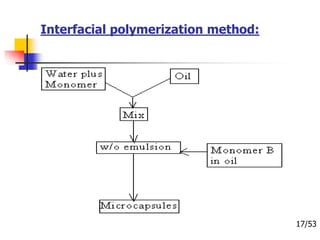
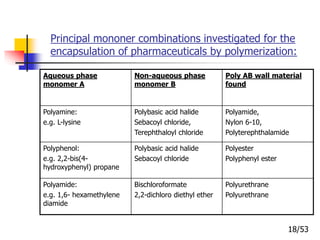
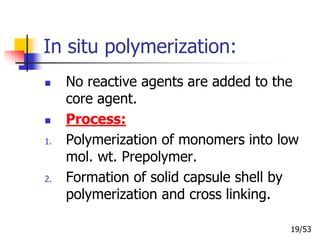
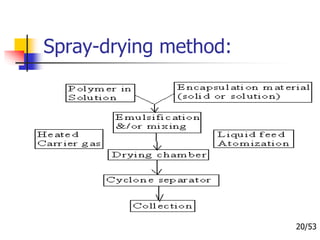
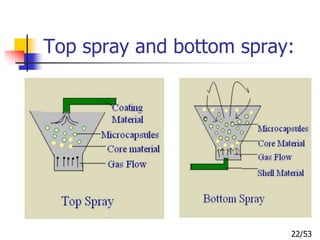
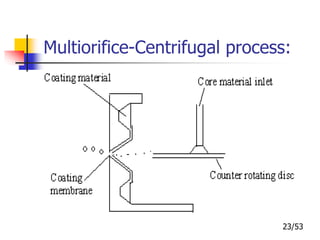
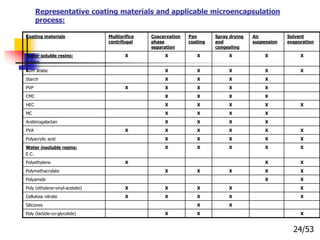
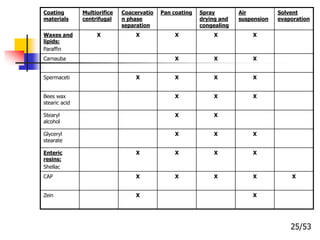
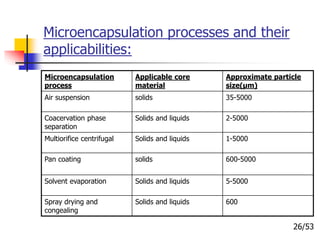
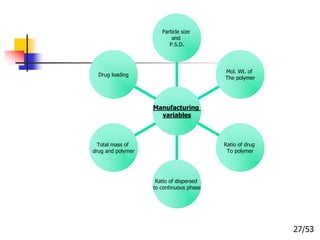
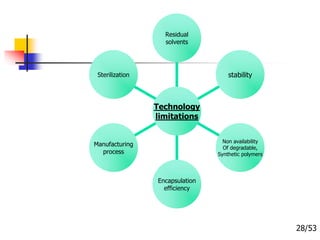
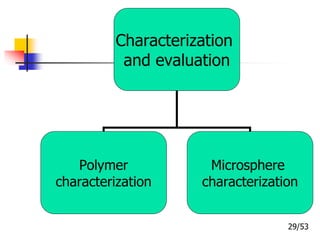
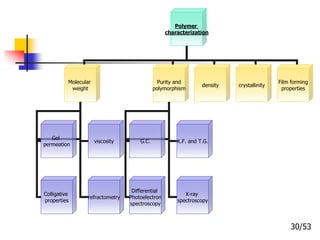
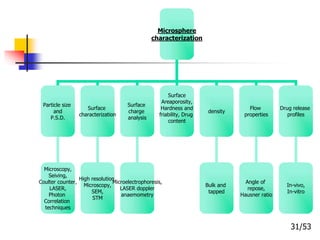
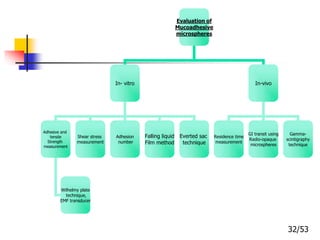
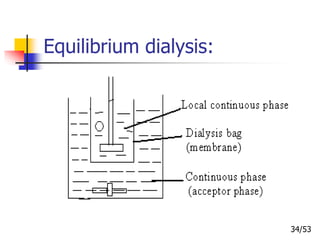
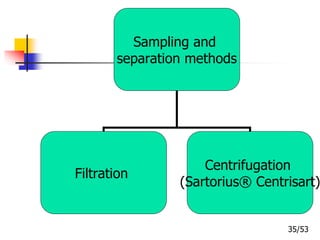
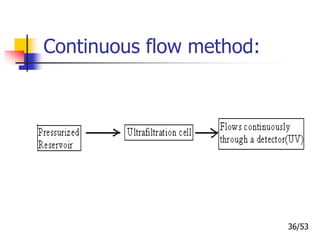
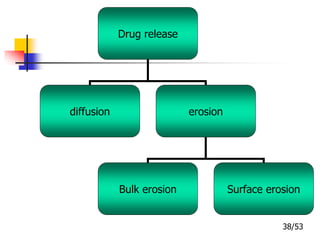
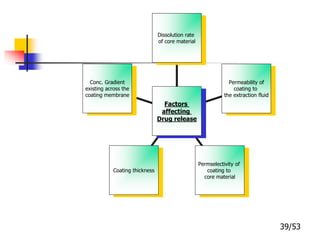
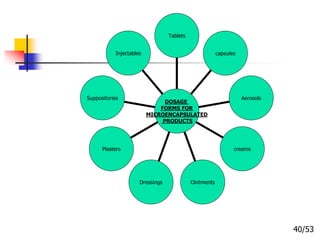
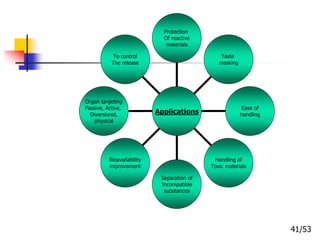
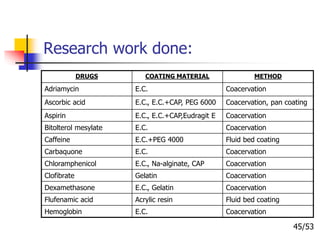
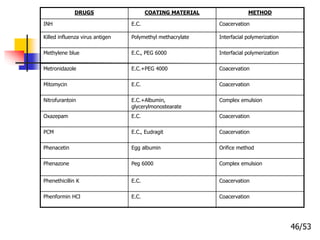
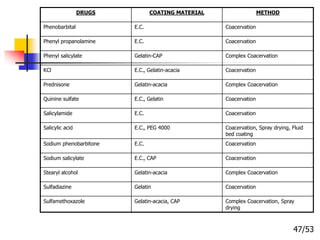
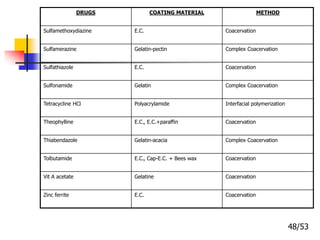
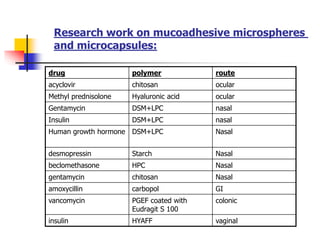
This document provides an overview of microencapsulation and microencapsulation drug delivery systems (MDDS). It discusses various microencapsulation processes including coacervation, solvent evaporation, polymer-polymer incompatibility, interfacial polymerization, and in situ polymerization. It also covers characterization techniques, drug release measurement methods, applications of microencapsulation in drug delivery and recent research advances in the field.