Chapter 6_Chemical-Equilibrium_Le Chateliers Principle-1.pdf
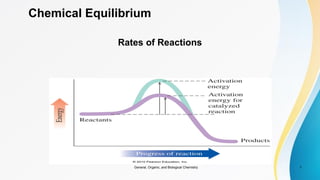
- 1. 1 Chemical Equilibrium Rates of Reactions General, Organic, and Biological Chemistry
- 2. 2 Collision Theory of Reactions A chemical reaction occurs when collisions between molecules have sufficient energy to break the bonds in the reactants molecules collide with the proper orientation bonds between atoms of the reactants (N2 and O2) are broken and new bonds (NO) form General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 3. 3 Collision Theory of Reactions (continued) A chemical reaction does not take place if the collisions between molecules do not have sufficient energy to break the bonds in the reactants molecules are not properly aligned General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 4. 4 Activation Energy The activation energy is the minimum energy needed for a reaction to take place upon proper collision of reactants General, Organic, and Biological Chemistry
- 5. 5 Reaction Rate and Temperature Reaction rate is the speed at which reactant is used up is the speed at which product forms increases when temperature rises because reacting molecules move faster, thereby providing more colliding molecules with energy of activation General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 6. 6 Reaction Rate and Concentration Increasing the concentration of reactants increases the number of collisions increases the reaction rate General, Organic, and Biological Chemistry
- 7. 7 Reaction Rate and Catalysts A catalyst • speeds up the rate of a reaction • lowers the energy of activation • is not used up during the reaction General, Organic, and Biological Chemistry
- 8. 8 Factors that Increase Reaction Rate General, Organic, and Biological Chemistry
- 9. 9 Learning Check 1: State the effect of each on the rate of reaction as: I) increases D) decreases N) no change A. increasing the temperature B. removing some of the reactants C. adding a catalyst D. placing the reaction flask in ice E. increasing the concentration of a reactant General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 10. 10 Learning Check 2: Indicate the effect of each factor listed on the rate of the following reaction as I) increases, D) decreases, or N) none: 2CO(g) + O2(g) 2CO2 (g) A. raising the temperature B. removing O2 C. adding a catalyst D. lowering the temperature General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 11. 11 Reversible Reactions In a reversible reaction, there are both forward and reverse reactions. • Suppose SO2 and O2 are present initially. As they collide, the forward reaction begins. 2SO2(g) + O2(g) 2SO3(g) • As SO3 molecules form, they also collide in the reverse reaction that forms reactants. This reversible reaction is written with a double arrow. forward 2SO2(g) + O2(g) 2SO3(g) reverse General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 12. 12 Chemical Equilibrium At equilibrium, the rate of the forward reaction becomes equal to the rate of the reverse reaction the forward and reverse reactions continue at equal rates in both directions General, Organic, and Biological Chemistry
- 13. 13 Chemical Equilibrium (continued) When equilibrium is reached, there is no further change in the amounts of reactant and product General, Organic, and Biological Chemistry
- 14. 14 Equilibrium At equilibrium, the forward reaction of N2 and O2 forms NO the reverse reaction of 2NO forms N2 and O2 the amounts of N2, O2, and NO remain constant N2(g) + O2(g) 2NO(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 15. 15 Learning Check 3: Write the forward and reverse reactions for the following: CH4(g) + 2H2S(g) CS2(g) + 4H2(g) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 16. 16 Learning Check 4: Complete with 1) equal 2) not equal 3) forward 4) reverse 5) changes 6) does not change A. Reactants form products in the ________ reaction. B. At equilibrium, the reactant concentration _______. C. When products form reactants, it is the _______ reaction. D. At equilibrium, the rate of the forward reaction is ______ to the rate of the reverse reaction. E. If the forward reaction is faster than the reverse, the amount of products ________. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 17. 17 Equilibrium Constants General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 18. 18 Equilibrium Constants For the reaction aA bB • The equilibrium constant expression, Kc, gives the concentrations of the reactants and products at equilibrium: Kc = [B]b = [Products] [A]a [Reactants] • The square brackets indicate the moles/liter of each substance. • The coefficients b and a are written as superscripts that raise the moles/liter to a specific power. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 19. 19 Guide to Writing the Kc Expression General, Organic, and Biological Chemistry
- 20. 20 Writing a Kc Expression Write the Kc expression for the following: STEP 1 Write the balanced equilibrium equation: 2CO(g) + O2(g) 2CO2(g) STEP 2 Write the product concentrations in the numerator and the reactant concentration in the denominator: Kc = [CO2] [products] [CO][O2] [reactants] STEP 3 Write the coefficients as superscripts: Kc = [CO2]2 [CO]2 [O2] General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 21. 21 Learning Check 5: Which of the following is the correctly written Kc expression for the reaction shown below? N2(g) + 3Cl2(g) 2NCl3(g) 1) [NCl3] 2) [N2][Cl2]3 [N2][Cl2] [NCl3]2 3) [NCl3]2 4) [NCl3]2 [N2]3 [Cl2] [N2][Cl2]3 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 22. 22 Heterogeneous Equilibrium In heterogeneous equilibrium, • Gases and solid and/or liquid states are part of the reaction. 2NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(g) • The concentration of solids and liquids is constant. • The Kc expression is written with only the compounds that are gases. Kc = [CO2][H2O] General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 23. 23 Learning Check 6: The Kc expression for the following reaction is: 2KClO3(s) 2KCl(s) + 3O2(g) 1) [KCl][O2] 2) [KCl]2[O2]3 [KClO3] [ KClO3]2 3) [KCl]2[O2]3 4) [O2]3 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 24. Guide to Calculating the Kc Value 24 General, Organic, and Biological Chemistry
- 25. 25 Example of Calculating Equilibrium Constants What is the Kc for the following reaction? H2(g) + I2(g) 2HI(g) Equilibrium concentrations: [H2] = 1.2 moles/L [I2] = 1.2 moles/L [HI] = 0.35 mole/L STEP 1 Write the Kc expression: Kc = [HI]2 [H2][I2] STEP 2 Substitute the equilibrium concentrations in Kc: Kc = [0.35]2 = 8.5 x 10-2 [1.2][1.2] General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 26. 26 Learning Check 7: Calculate the Kc for the following reaction: CH4(g) + H2O(g) CO(g) + 3H2(g) with the following equilibrium concentrations: [CH4] = 0.13 M [H2O] = 0.53 M [CO] = 0.81 M [H2] = 0.28 M 1) 0.26 2) 3.3 3) 3.9 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 27. 27 Reaching Chemical Equilibrium A container initially filled with SO2(g) and O2(g) or only SO3(g) contains mostly SO3(g) and small amounts of O2(g) and SO3(g) at equilibrium reaches equilibrium in both situations General, Organic, and Biological Chemistry
- 28. 28 Equilibrium Can Favor Product If equilibrium is reached after most of the forward reaction has occurred, the system favors the products General, Organic, and Biological Chemistry
- 29. 29 Equilibrium with a Large Kc At equilibrium, • a reaction with a large Kc produces a large amount of product; very little of the reactants remain Kc = [NCl3]2 = 3.2 x 1011 [N2][Cl2]3 • a large Kc favors the products N2(g) + 3Cl2(g) 2NCl3(g) When this reaction reaches equilibrium, it will essentially consist of the product NCl3. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 30. 30 Equilibrium Can Favor Reactant If equilibrium is reached when very little of the forward reaction has occurred, the reaction favors the reactants General, Organic, and Biological Chemistry
- 31. 31 Equilibrium with a Small Kc At equilibrium, • a reaction that produces only a small amount of product has a small Kc Kc = [NO]2 = 2.3 x 10-9 [N2][O2] • a small Kc favors the reactants N2(g) + O2(g) 2NO(g) When this reaction reaches equilibrium, it will essentially consist of the reactants N2 and O2. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 32. 32 Summary of Kc Values A reaction that favors products has a large Kc with about equal concentrations of products and reactants has a Kc close to 1 that favors reactants has a small Kc General, Organic, and Biological Chemistry
- 33. Summary of Kc Values (continued) 33 General, Organic, and Biological Chemistry
- 34. 34 Large and Small Kc Values General, Organic, and Biological Chemistry
- 35. 35 Learning Check 8: For each Kc, indicate whether the reaction at equilibrium contains mostly of (R) reactants or (P) products. __A. H2(g) + F2(g) 2HF(g) Kc = 1 x 1095 __B. 3O2(g) 2O3(g) Kc = 1.8 x 10-7 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 36. Guide to Using the Kc Value 36 General, Organic, and Biological Chemistry
- 37. 37 Using Kc to Solve for an Equilibrium Concentration At equilibrium, the reaction PCl5(g) PCl3(g) + Cl2(g) has a Kc of 4.2 x 10–2 and contains [PCl3] = [Cl2] = 0.10 M. What is the equilibrium concentration of PCl5? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 38. 38 Using Kc to Solve for an Equilibrium Concentration (continued) STEP 1 Write the Kc expression: Kc = [PCl3][Cl2] [PCl5] STEP 2 Solve for the unknown concentration: [PCl5] = [PCl3][Cl2] Kc STEP 3 Substitute the known values and solve: [PCl5] = [0.10][0.10] = 0.24M 4.2 x 10–2 STEP 4 Check answer by placing concentrations in Kc: Kc = [0.10][0.10] = 4.2 x 10–2 [0.24]
- 39. 39 Learning Check 9: The Kc is 2.0 for the reaction: 2NOBr(g) 2NO(g) + Br2(g) If the equilibrium concentrations are [NOBr] = 0.50 M and [NO] = 0.80 M, what is the equilibrium concentration of Br2? 1) 0.39 M 2) 0.78 M 3) 1.3 M General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 40. 40 Le Chatelier’s Principles: Changing Equilibrium Conditions: Le Châtelier’s Principle General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 41. 41 Le Châtelier’s Principle Le Châtelier’s principle states that • any change in equilibrium conditions upsets the equilibrium of the system • a system at equilibrium under stress will shift to relieve the stress • there will be a change in the rate of the forward or reverse reaction to return the system to equilibrium General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 42. 42 Adding Reactant For the reaction A + B C at equilibrium, adding more A upsets the equilibrium the rate of forward reaction increases to re-establish Kc A + B C General, Organic, and Biological Chemistry
- 43. 43 Effect of Adding Reactant Consider the following reaction at equilibrium. H2(g) + F2(g) 2HF(g) • If more reactant (H2 or F2) is added, there is an increase in the number of collisions. • The rate of the forward reaction increases and forms more HF product until new equilibrium concentrations equal Kc again. • The effect of adding a reactant shifts the equilibrium toward the products. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 44. 44 Effect of Adding Product Consider the following reaction at equilibrium. H2(g) + F2(g) 2HF(g) • When more HF is added, there is an increase in collisions of HF molecules. • The rate of the reverse reaction increases and forms more H2 and F2 reactants. • The effect of adding a product shifts the equilibrium toward the reactants. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 45. 45 Adding Reactant or Product The equilibrium shifts toward • products when H2(g) or F2(g) is added • reactants when HF(g) is added H2(g) + F2(g) 2HF(g) Add H2 or F2 Add HF General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 46. 46 Effect of Removing Reactant Removing reactant, H2(g) or F2(g), from the following reaction at equilibrium: H2(g) + F2(g) 2HF(g) • decreases the collisions between reactants • decreases the rate of the forward reaction • shifts the equilibrium toward the reactants H2(g) + F2(g) 2HF(g) Remove H2 or F2 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 47. 47 Effect of Removing Product Removing HF(g) from the following reaction at equilibrium: H2(g) + F2(g) 2HF(g) • decreases the number of collisions between products • decreases the rate of the reverse reaction • shifts equilibrium toward the products H2(g) + F2(g) 2HF(g) Remove HF General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 48. 48 Concentration Changes and Equilibrium General, Organic, and Biological Chemistry
- 49. Effect of a Catalyst Adding a catalyst • lowers the activation energy of the forward reaction • increases the rate of the forward reaction • lowers the activation energy of the reverse reaction • increases the rate of the reverse reaction • decreases the time to reach equilibrium • has no effect on the equilibrium 49 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 50. 50 Learning Check 10: Predict any shift in equilibrium for each of the following changes on the reaction NH4HS(s) H2S(g) + NH3(g) 1) to products 2) to reactants 3) no change A. H2S(g) is added. B. NH4HS(s) is added. C. NH3(g) is removed. D. A catalyst is added. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 51. 51 Effect of Decreasing the Volume When a reaction at equilibrium contains different numbers of moles of reactants than products, a decrease in volume • increases the concentrations (mole/L), upsetting the equilibrium • shifts the equilibrium toward the fewer number of moles N2(g) + 3H2(g) 2NH3(g) Decrease volume More moles Fewer moles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 52. 52 Volume Decrease and Equilibrium A volume decrease shifts the equilibrium toward the side (A) with the smaller number of moles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 53. 53 Effect of Increasing the Volume When a reaction at equilibrium contains different numbers of moles of reactants than products, an increase in volume • decreases the concentrations (mole/L), upsetting the equilibrium • shifts the equilibrium toward the greater number of moles N2(g) + 3H2(g) 2NH3(g) Increase volume More moles Fewer moles
- 54. 54 Volume Increase and Equilibrium A volume increase shifts the equilibrium toward the side (B and C) with the greater number of moles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 55. Effect of Pressure • Affects gases only. • For unequal number of moles of reactants and products, if pressure is increased, the equilibrium will shift to reduce the number of particles. • For equal number of moles of reactants and products, no shift occurs. 2NO2 (g) N2O4 (g)
- 56. Ex: Effect of Pressure 2NO2 (g) N2O4 (g) 2 moles 1 mole Stress: increasing the pressure Shift: to the right (side of less moles) Stress: decreasing the pressure Shift : to the left (side of more moles )
- 57. 57 Heat and Endothermic Reactions For an endothermic reaction at equilibrium, • a decrease in temperature (T) removes heat, and the equilibrium shifts toward the reactants • an increase in temperature adds heat, and the equilibrium shifts toward the products. CaCO3(s) + 133 kcal CaO(s) + CO2(g) Decrease T Increase T General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 58. 58 Equilibrium Shift in Endothermic Reactions with Temperature General, Organic, and Biological Chemistry
- 59. 59 Heat and Exothermic Reactions For an exothermic reaction at equilibrium, • a decrease in temperature removes heat, and the equilibrium shifts toward the products • an increase in temperature adds heat, and the equilibrium shifts toward the reactants. N2(g) + 3H2(g) 2NH3(g) + 22 kcal Decrease T Increase T General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 60. 60 Equilibrium Shifts with Temperature in Exothermic Reactions General, Organic, and Biological Chemistry
- 61. 61 Learning Check 11: Indicate if each change on a reaction at equilibrium shifts 2NO2(g) + heat 2NO(g) + O2(g) 1) towards products 2) towards reactants 3) no change A. adding NO(g) F. decreasing pressure B. adding O2(g) G. decreasing the tempt. (Endo) C. raising the temperature(Exo) D. removing O2(g) E. increasing the volume General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 62. 62 Chemical Equilibrium Equilibrium in Saturated Solutions General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 63. 63 Saturated Solution A saturated solution contains the maximum amount of dissolved solute contains solid solute is an equilibrium system: rate of dissolving = rate of recrystallization solid ions in solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 64. 64 Solubility Product Constant The solubility product constant for a saturated solution gives the ion concentrations at constant temperature is expressed as Ksp does not include the solid, which is constant Fe(OH)2(s) Fe2+(aq) + 2OH−(aq) Ksp = [Fe2+] [OH−]2 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 65. 65 Seatwork: Write the Ksp expression for each of the following: A. Ag2CrO4(s) --> 2 Ag+(aq) + CrO4 2-(aq) B. BaSO4(s) --> Ba2+(aq) + SO4 2-(aq) C. PbCl2(s) --> Pb2+(aq) + 2 Cl-(aq) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 66. 66 Guide to Calculating Ksp General, Organic, and Biological Chemistry
- 67. 67 Example of Calculating Solubility Product Constant Calculate the Ksp of PbSO4 (solubility 1.4 x 10–4 M). STEP 1 Write the equilibrium equation for dissociation: PbSO4(s) Pb2+(aq) + SO4 2−(aq) STEP 2 Write the Ksp expression: Ksp = [Pb2+][SO4 2−] STEP 3 Substitue molarity values and calculate: Ksp = (1.4 x 10–4) x (1.4 x 10–4) = 2.0 x 10–8 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 68. 68 Examples of Solubility Product Constants General, Organic, and Biological Chemistry
- 69. 69 Learning Check 13: What is the Ksp value of PbF2 if the solubility at 25 C is 2.6 x 10–3 M? 1) 1.4 x 10–5 2) 6.8 x 10–6 3) 7.0 x 10–8 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 70. 70 Molar Solubility (S) The molar solubility (S) is the number of moles of solute that dissolve in 1 L of solution determined from the formula of the salt calculated from the Ksp General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 71. 71 Calculating Molar Solubility (S) General, Organic, and Biological Chemistry
- 72. 72 Solubility Calculation Determine the solubility (S)2 of SrCO3 (Ksp = 5.4 x 10–10). STEP 1 Write the equilibrium equation for dissociation: SrCO3(s) Sr2+(aq) + CO3 2−(aq) STEP 2 Write the Ksp expression: Ksp = [Sr2+][CO3 2−] STEP 3 Substitute S for the molarity of each ion into Ksp: Ksp = [Sr2+][CO3 2−] = [S][S] = S2 = 5.4 x 10−10 STEP 4 Calculate the solubility, S: S = = 2.3 x 10−5 M -10 5.4 10 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.
- 73. 73 Seatwork: 1. Calculate the solubility (S) of CaF2, if the Ksp =3.9 x 10 -11. 2. Calculate the solubility (S) of PbSO4 , if the Ksp = 1.6 x 10–8. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.