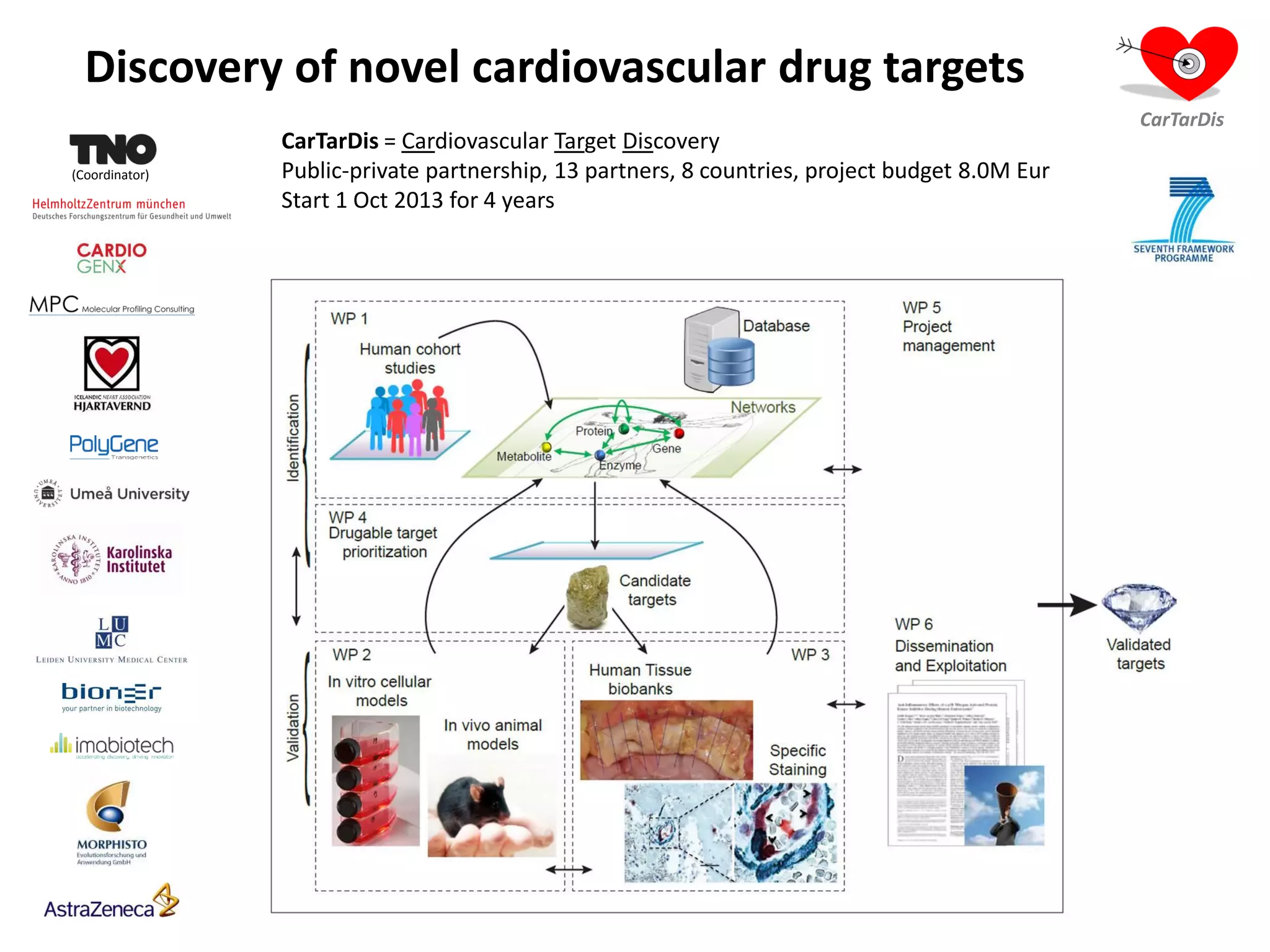
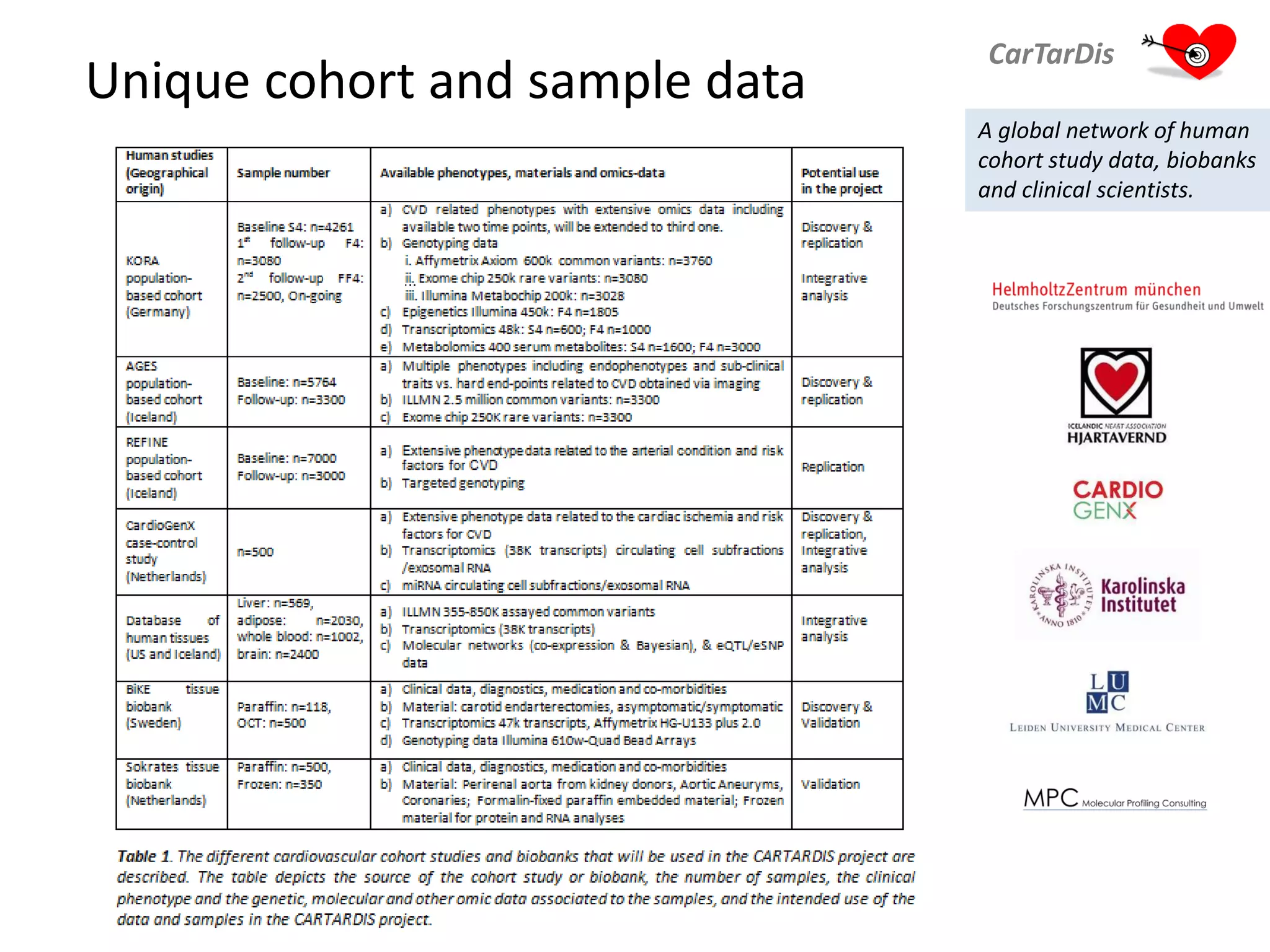
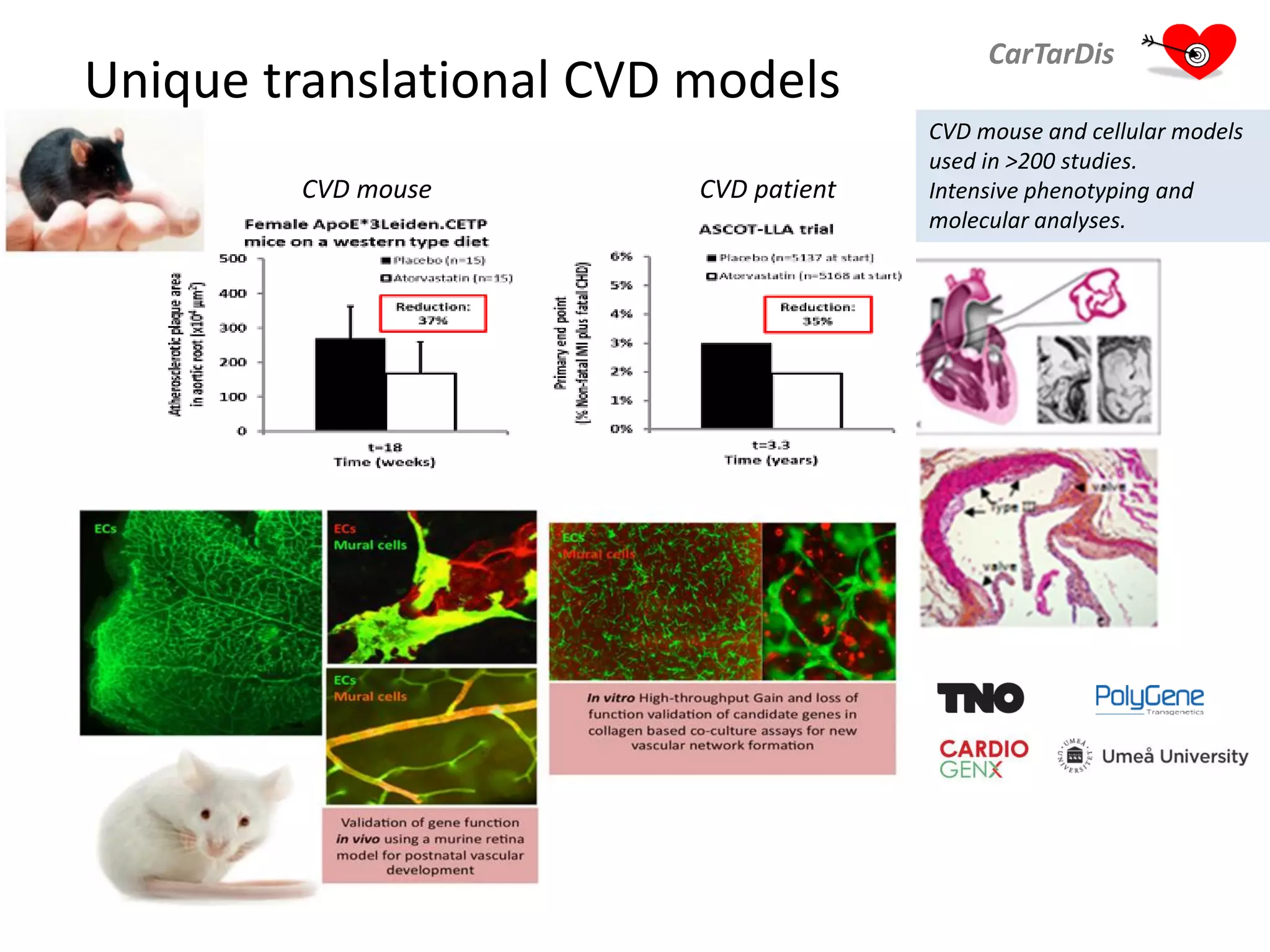
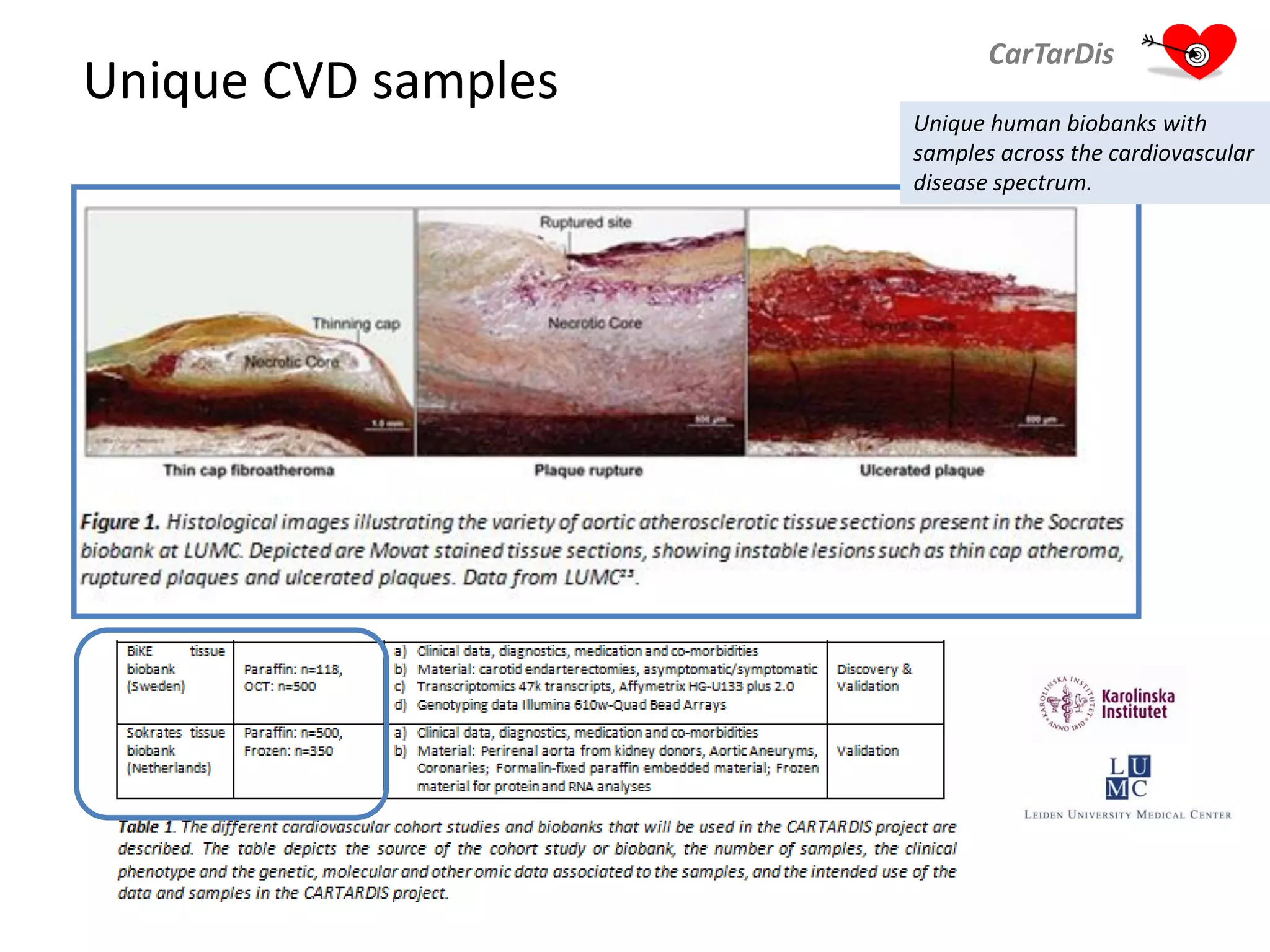
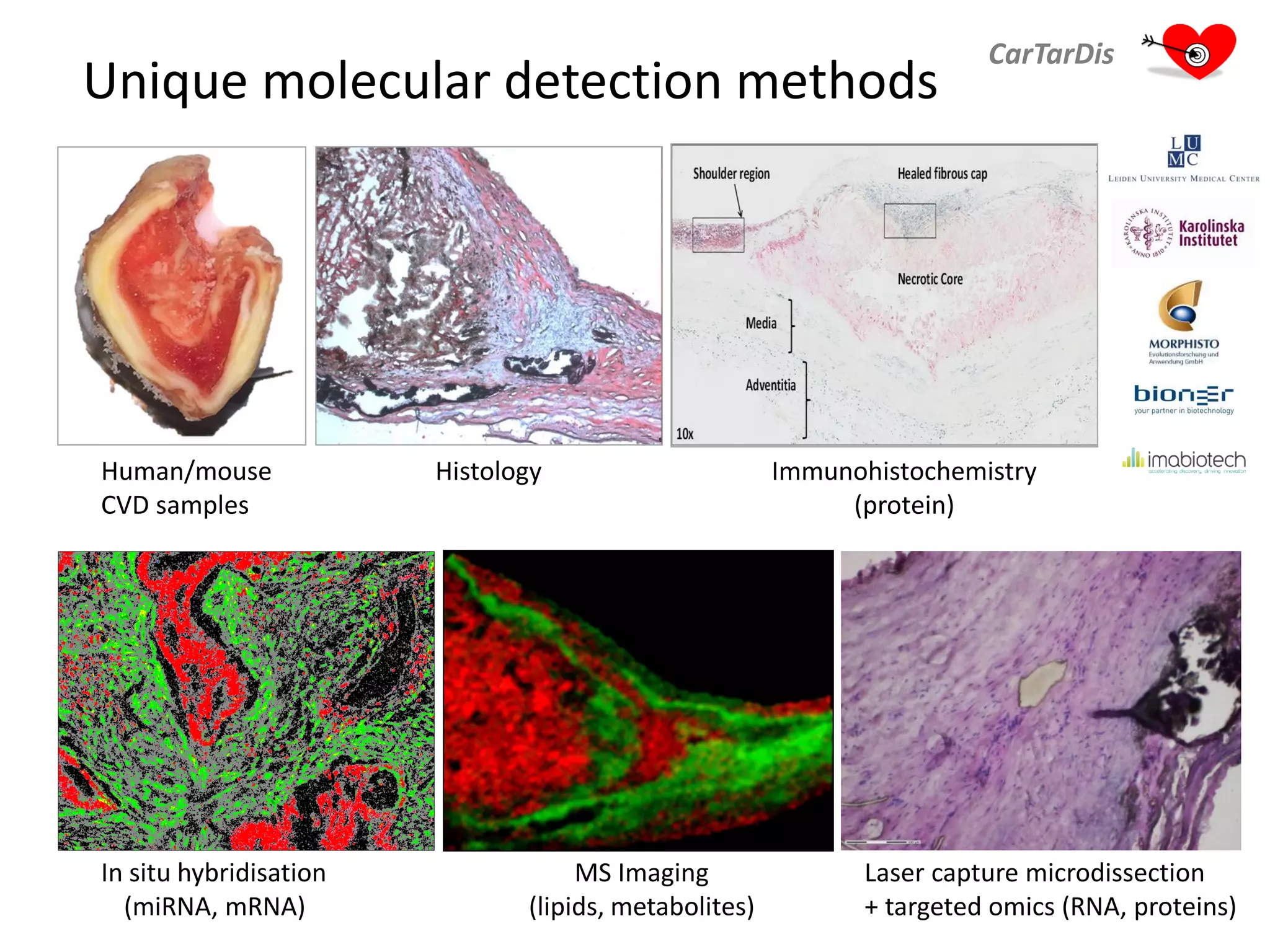
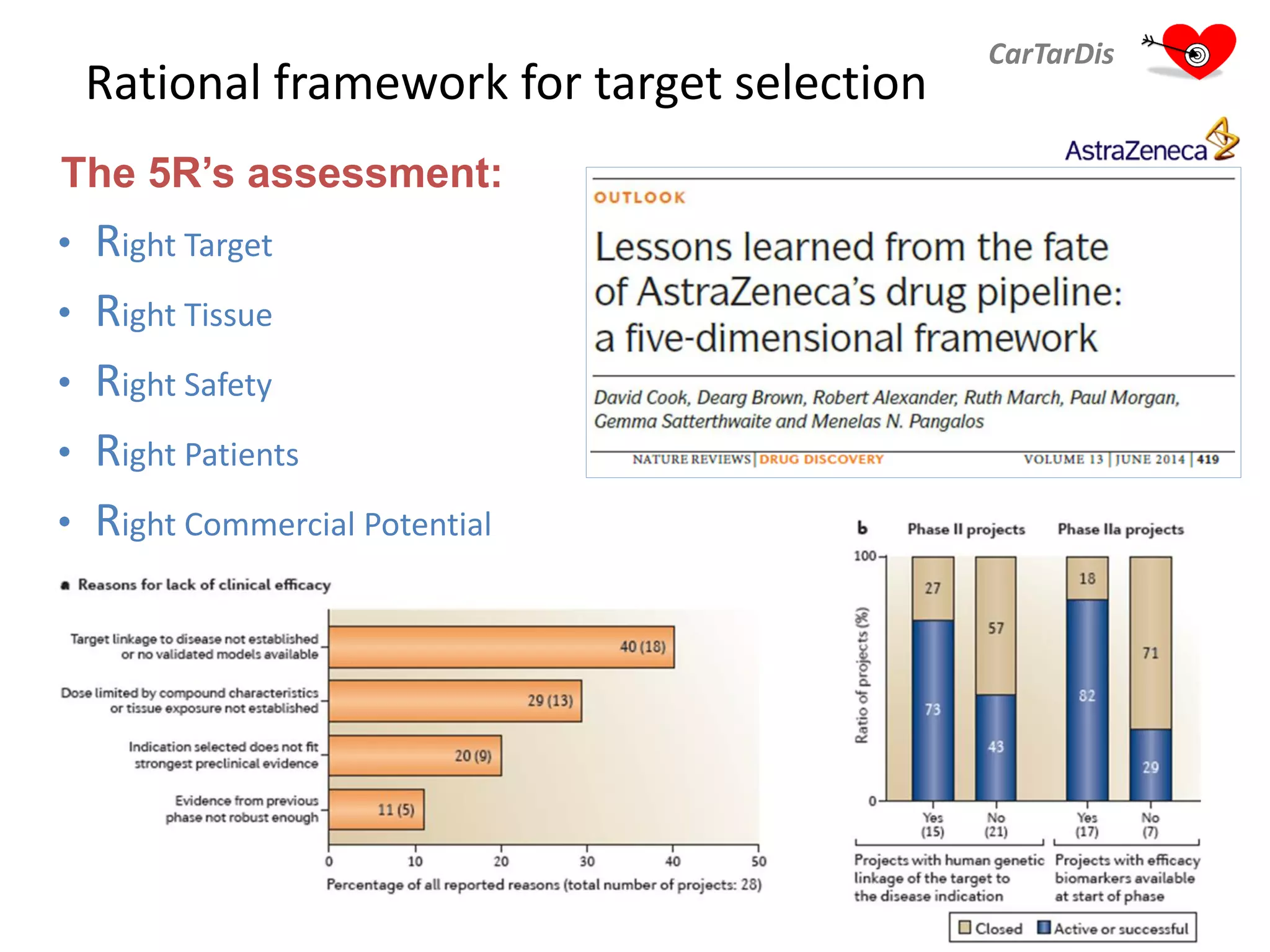
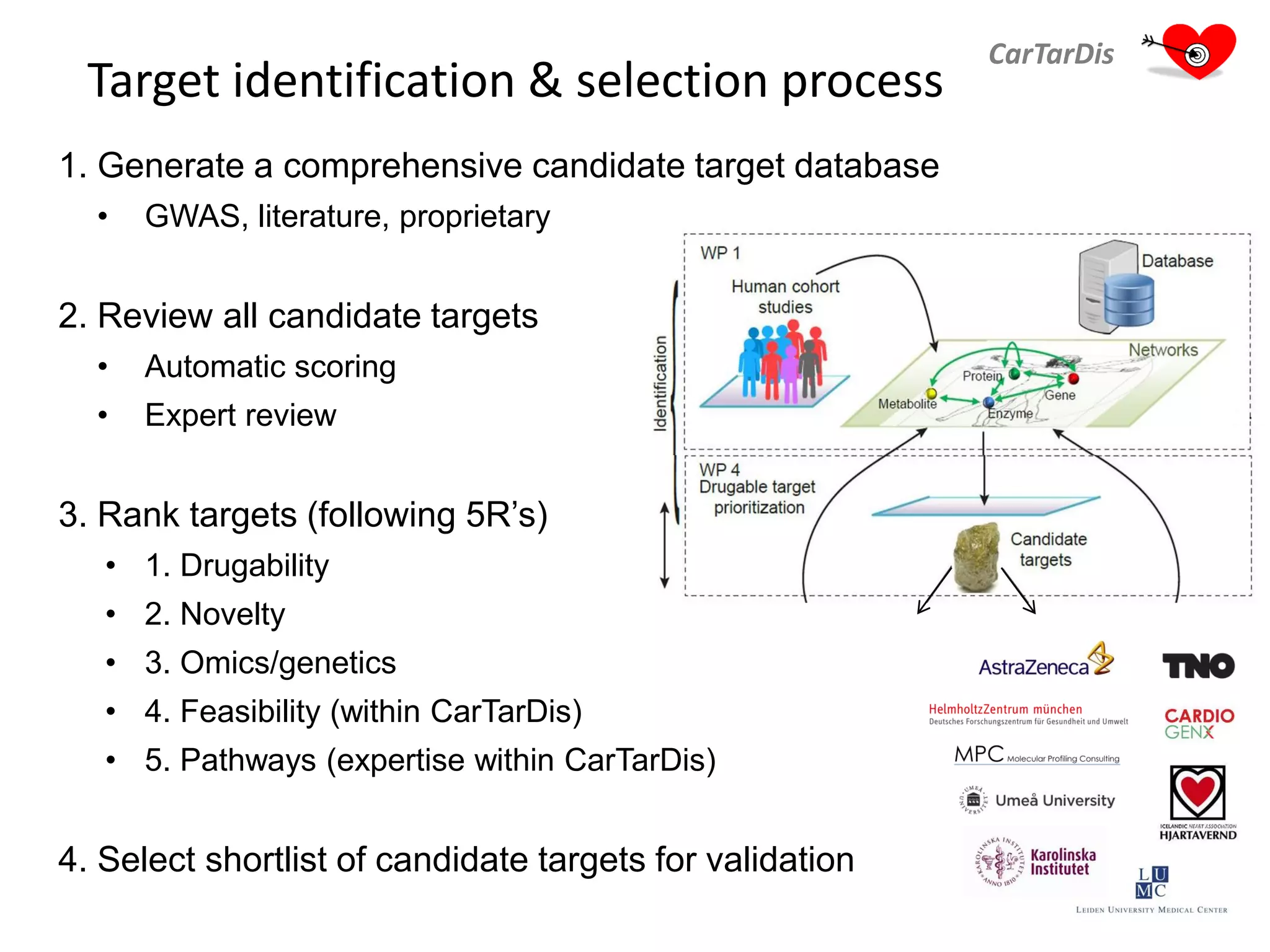
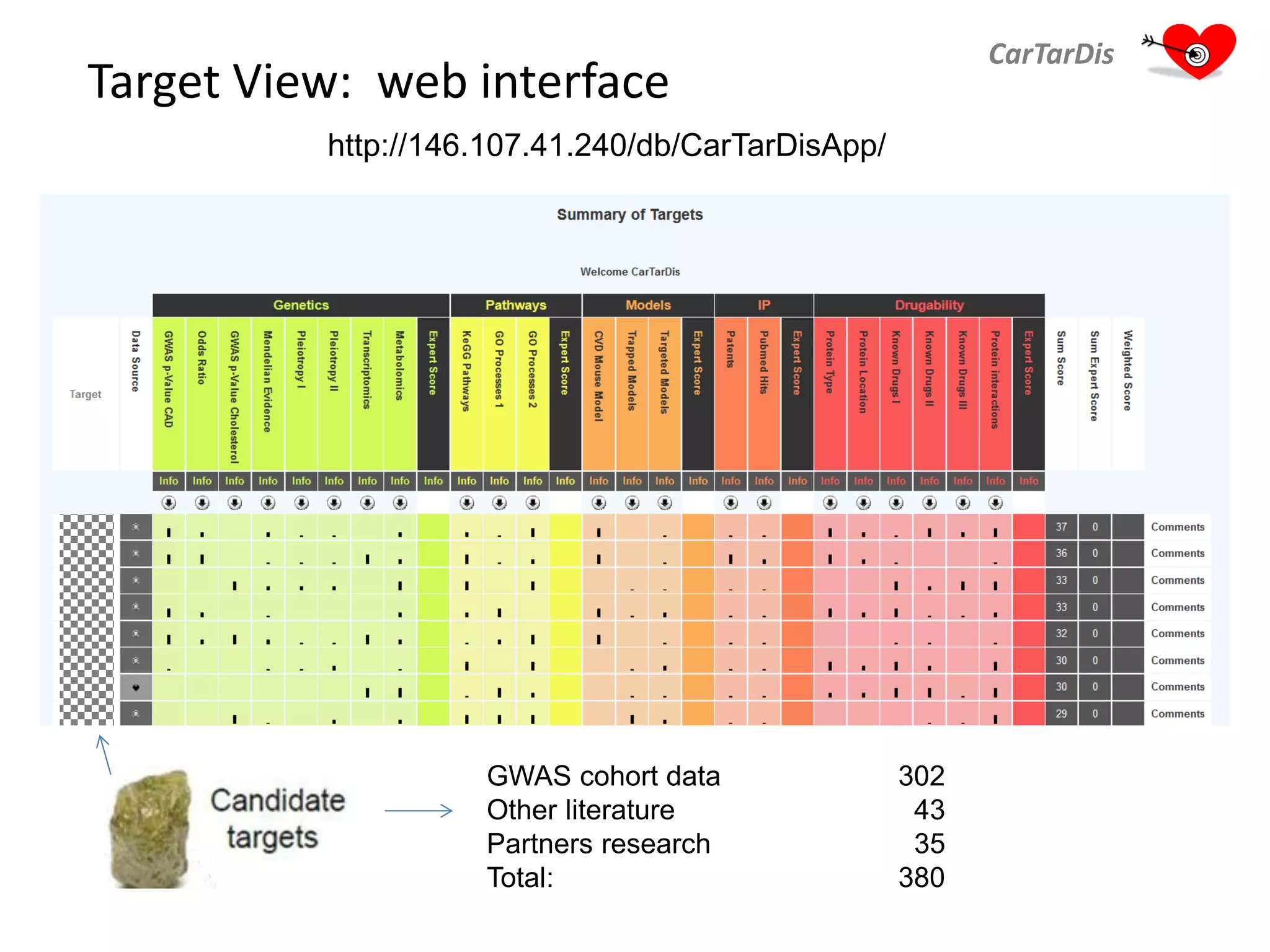
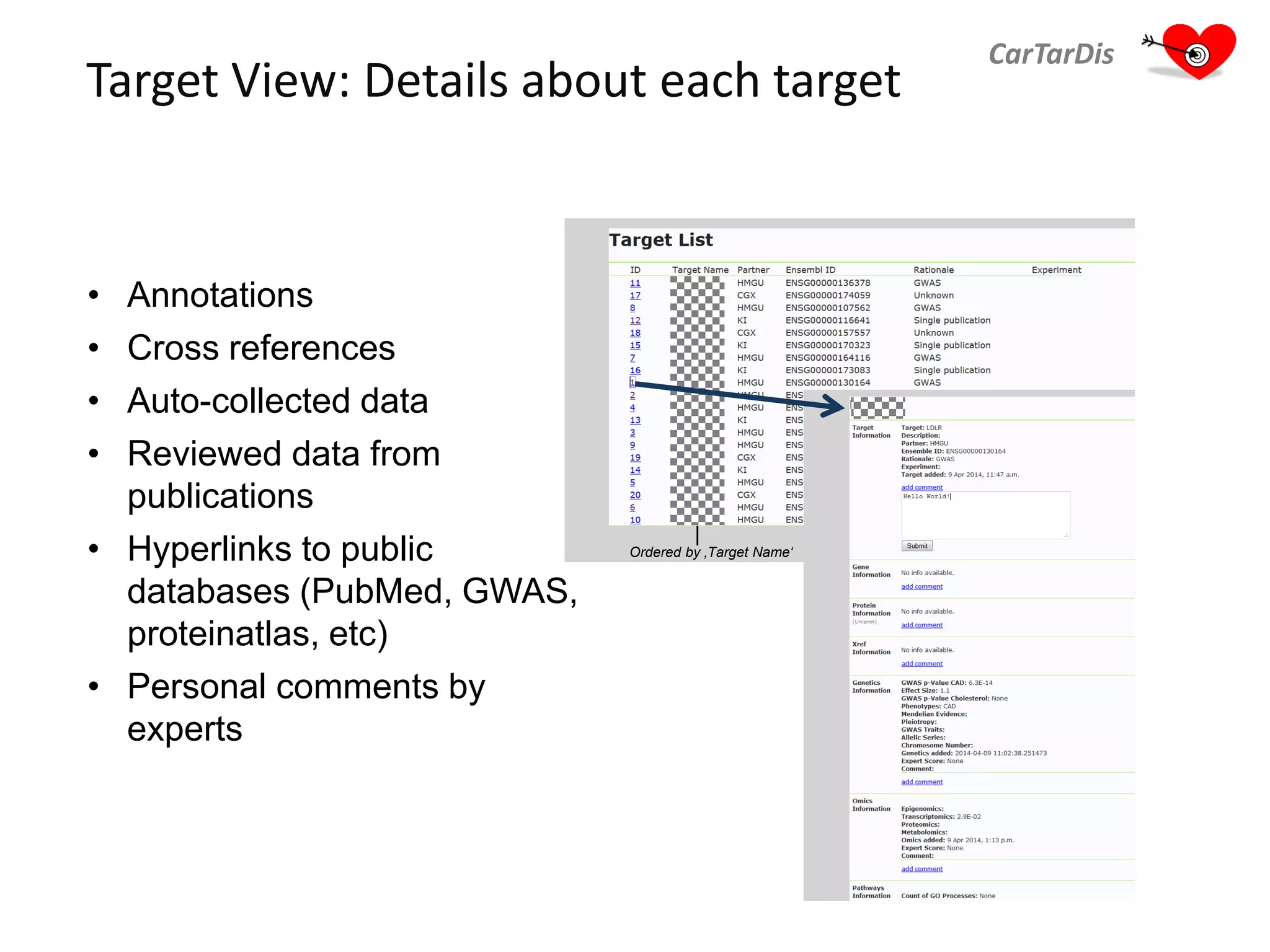
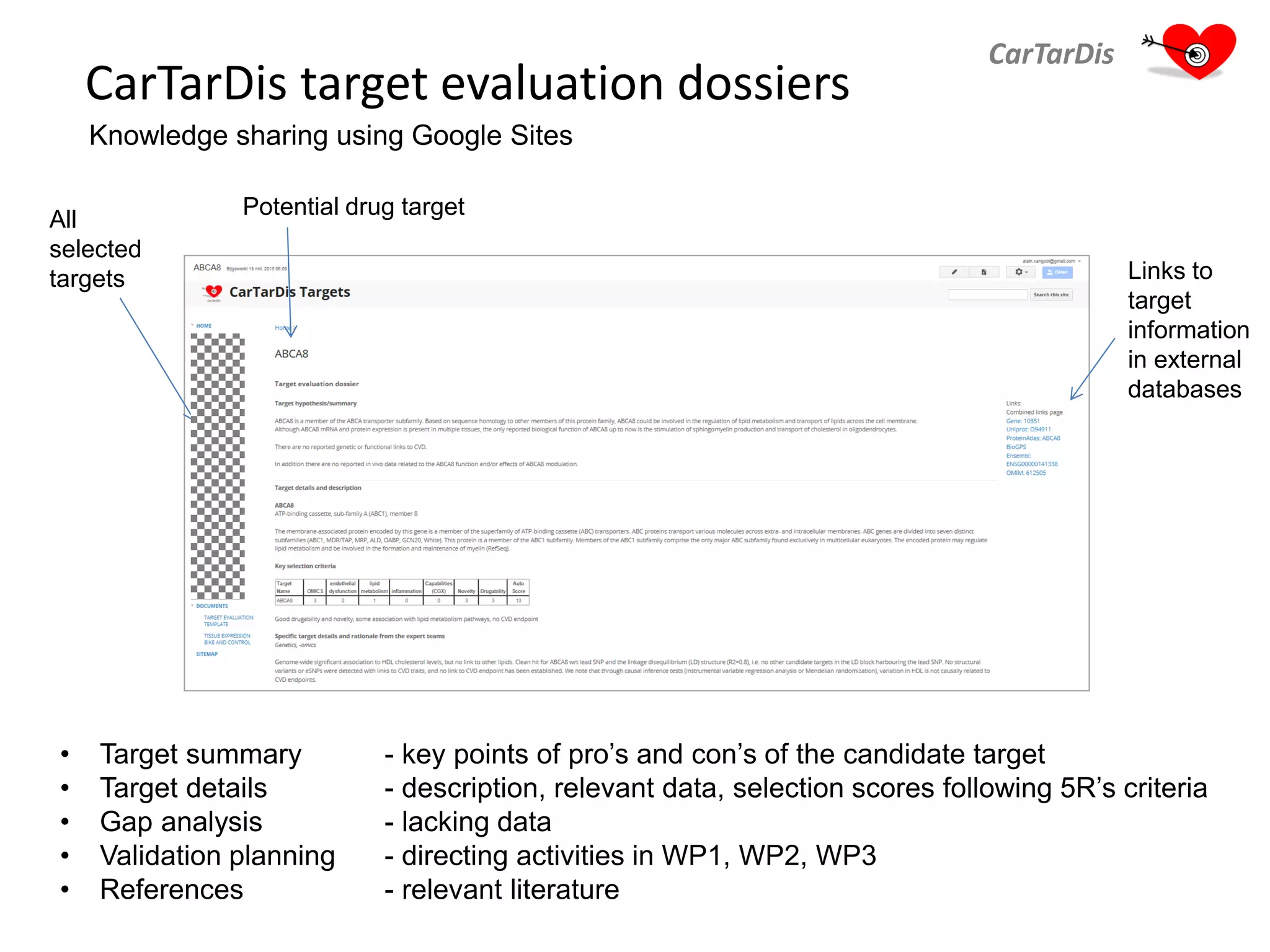
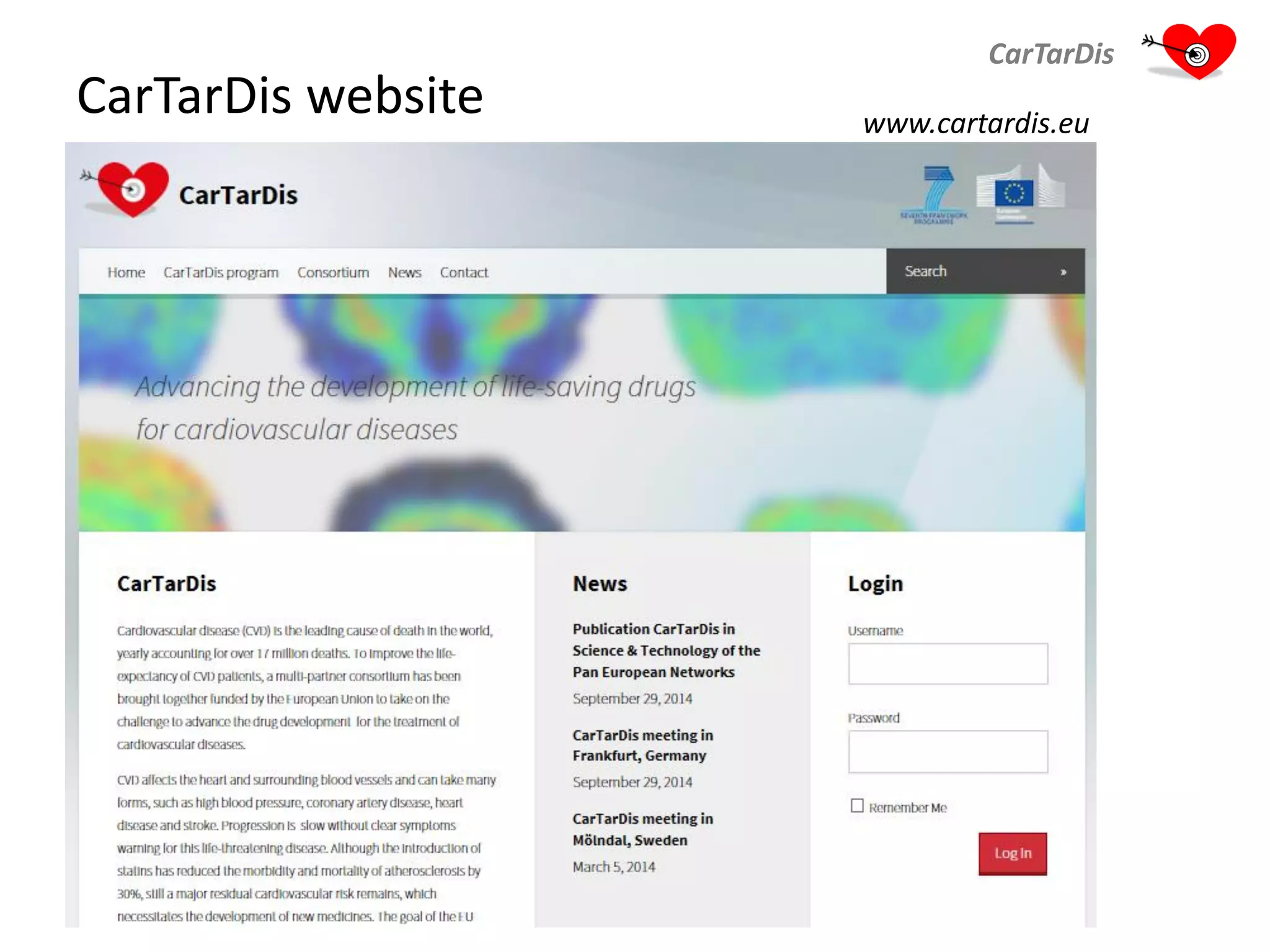
The document describes CarTarDis, a 4-year public-private partnership with 13 partners from 8 countries and a budget of 8.0M Euros that began on October 1, 2013. The project aims to discover novel cardiovascular drug targets through a strong interdisciplinary team with expertise in cardiovascular disease biology, drug discovery, translational medicine, omics technologies, and more. CarTarDis will utilize unique human cohort data, biobanks, CVD models and samples to generate a candidate target database, review targets, validate a shortlist, and create online dossiers to share knowledge on selected targets. The goal is to prioritize 11 targets for further validation activities to identify new treatments for cardiovascular disease.