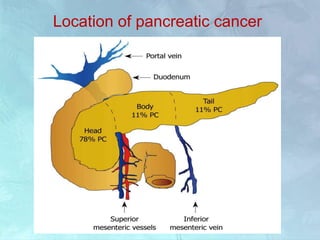
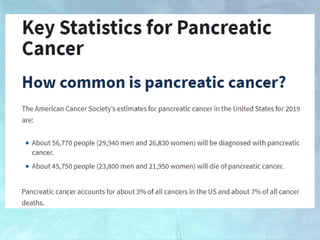
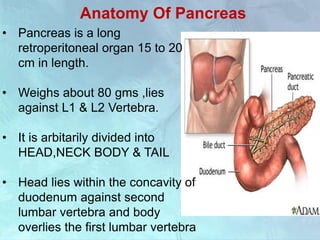
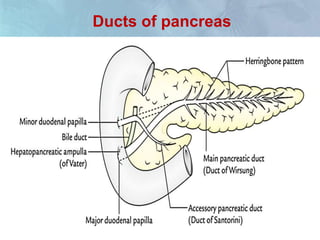
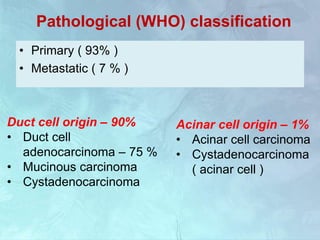
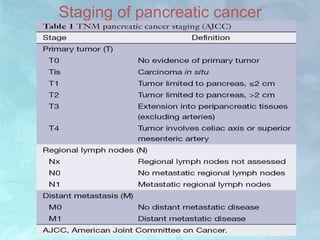
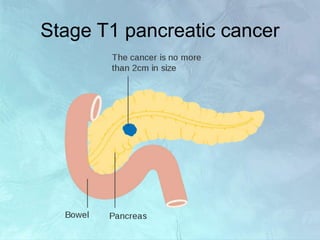
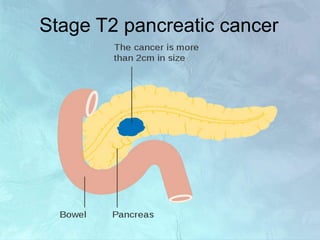
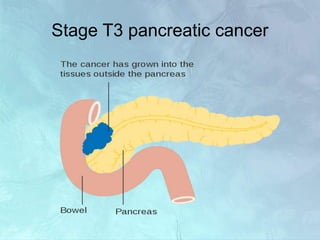
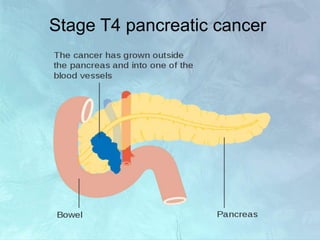
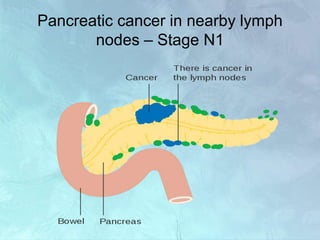
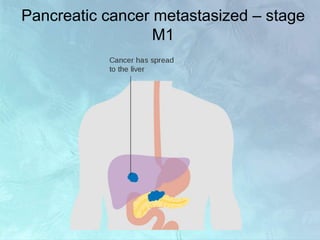
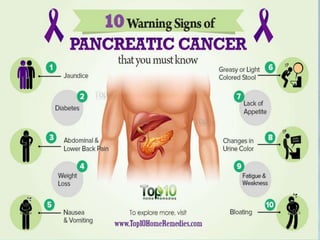
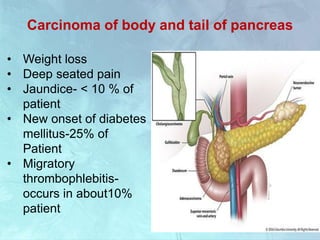
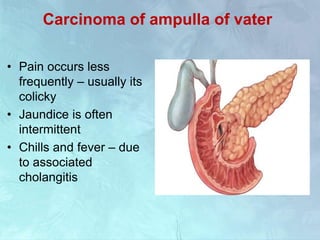
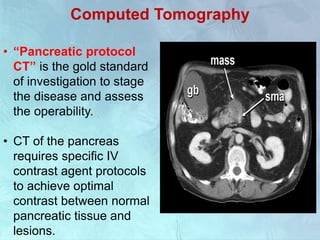
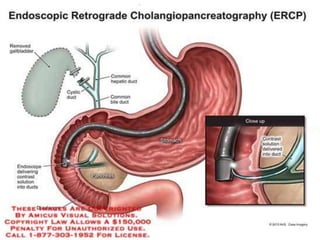
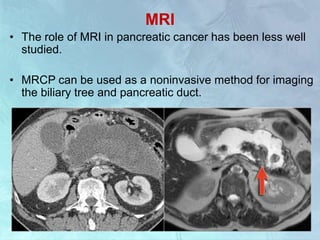
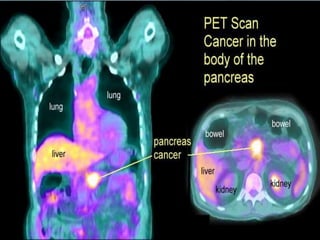
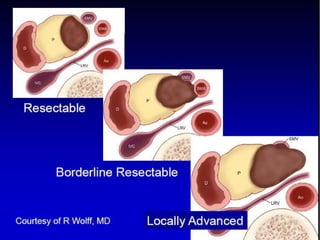
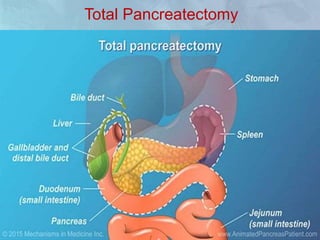
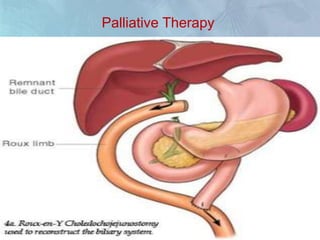
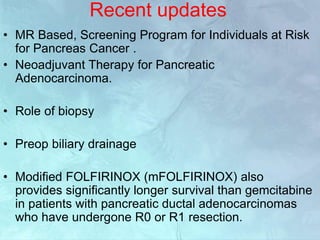
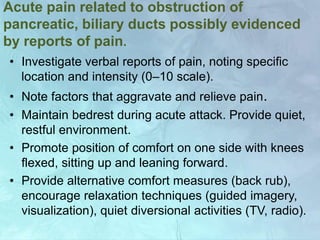
A 63-year-old male presented with epigastric pain radiating to the back, abdominal distention, vomiting and jaundice. Laboratory tests showed elevated bilirubin and liver enzymes. CT scan revealed a 3.5 cm x 3.7 cm pancreatic head mass and multiple liver nodules, indicating stage IV pancreatic cancer with liver metastasis. The patient was diagnosed with advanced pancreatic cancer based on imaging and laboratory findings.