Blue PTS of The World Characters Horizons.ppt
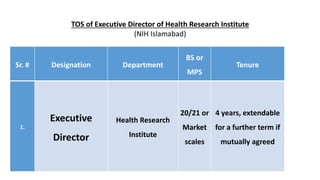
- 1. TOS of Executive Director of Health Research Institute (NIH Islamabad) Sr. # Designation Department BS or MPS Tenure 1. Executive Director Health Research Institute 20/21 or Market scales 4 years, extendable for a further term if mutually agreed
- 2. Eligibility criterion as previously advertised: Executive Director Health Research Institute 1 Merit i. MBBS, MD or equivalent degree recognised by PMC. And M.Phil/MS/MPH/FCPS in Public Health / Community Medicine / Epidemiology/ Biostatistics/Pathology, or Dip. Am Board (Internal medicine or Preventive Medicine or infectious diseases) Preferably: i. PhD or post-graduate training from recognized and accredited university in Pakistan or abroad in the relevant field for 2 years ii. Track record and ability to attract research grants, and conduct research projects or establish a research program. iii. At least ten (10) relevant peer-reviewed research papers published in national or international indexed and high impact journals during preceding 5 years. iv. Minimum 12 years of post-qualification experience in relevant field and research with experience in the applied public health profession or 3 years administrative experience as chief of a Division of National Institute of Health or as head of an organization / institute/department of similar mandate.
- 3. Scale: Basic Scale 20/21 or market scales Tenure: 4 years, extendable for a further term if mutually agreed Experience: Experience means post qualification experience gained in a regular full-time paid job or in an organization On Promotion: service 15 years with minimum 7 years in BS 19 On direct induction/deputation: Minimum 12 years of experience in relevant field and research with experience in the applied public health profession or 3 years administrative experience as chief of a Division of National Institute of Health or as head of an organization / institute/department of similar mandate. Job Description a. Shall be responsible to promote and coordinate health research in Pakistan and act as custodian of public research funds. b. The Institute shall perform functions as directed and assigned by the Federal Government or the Board c. To promote, organize and conduct scientific research in the field of health and alternate medicine including allopathy, homeopathy, herbal, unani, ayurvedic and traditional medicine: Evaluate, perform research, analyze and advise on environmental hazards and safety;
- 4. i. Ensure ethical compliance of medical and health related research to establish national and international standards. ii. Provide financial support for health research through grants and fellowships. iii. Evaluate submitted research proposals for scientific quality, ethical compliance and validity of outcomes, in order to grant funds for the research purpose. iv. Oversee research projects funded by NIH for scientific integrity and ethical compliance. Establish National Ethical/Bioethical Committee to coordinate and promote ethical practices as necessary of biomedical research in Pakistan. v. Advise Federal and provincial Governments and any other relevant body or organization on matters related health research with knowledge of Chief Executive Officer. vi. Establish strengthen and expand bioinformatics for support of research. vii. Establish linkages with national and international research institutions, bodies and organizations as well as relevant community groups and agencies. vii. To develop financial and other resources for its functions and for support of research d. Act as a custodian of all records and official seal of institute. e. Oversee administration, Control and supervision of clinical trial wing. f. Oversee training wing for international/National trainings in fellowships, diplomas, certification etc in health research. g. Oversee appointment officers and other employees of institute as may be delegated by the board in the prescribed manner.
- 5. h. Supervise and control the administration of HRI and its HR, including disciplinary action against employee in a manner prescribed by the board. i. Authorize payments and incur expenditures in accordance with the financial rules as prescribed. j. Execute deeds, documents and contracts on behalf of center in a manner delegated by the board. k. Propose budgetary estimates of HRI in consultation with the chief financial officer for approval by the Board of Governors (BoGs) of NIH. l. Take steps for controlling and implementation of the functions of the HRI, decisions of the BoGs, CEO, working groups, committees and sub-committees. m. Prepare Annual Health Report related to the Dept and present to the BoGs for approval. n. Negotiate grants, funding and receive donations for the benefit of the center generally or for specific projects.
