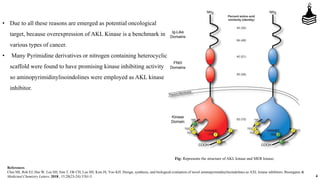
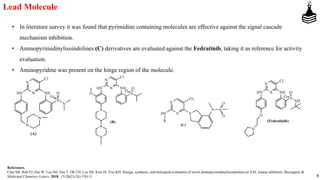
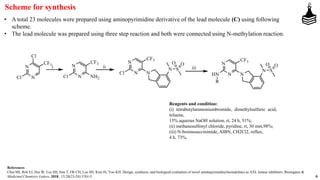
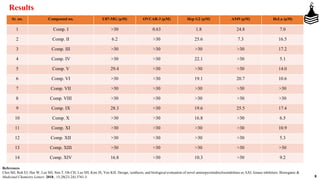
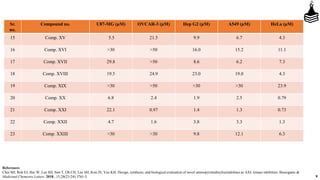
This document discusses the role of aminopyrimidinylisoindolines as AXL kinase inhibitors in cancer therapy, highlighting their potential in targeting overexpressed kinases linked to various cancers. It details the synthesis, clinical evaluation, and results of novel compounds, identifying one particularly effective compound (comp. xxi) with low IC50 values against AXL family kinases. The conclusion suggests that comp. xxi can serve as a lead molecule for further kinase inhibition studies.