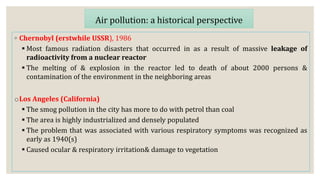
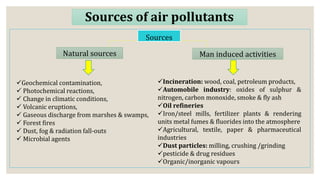
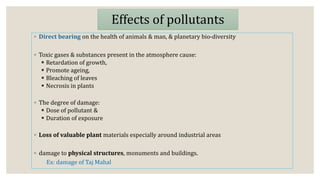
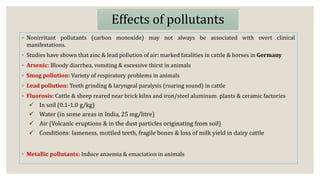
This document discusses air pollution and the composition of the atmosphere. It describes the different zones of the atmosphere and factors that influence air pollution, including meteorological conditions. Several historical air pollution disasters are summarized that resulted from high levels of sulfur compounds and other pollutants. Air pollutants are classified by their source and effects. Pollution within animal houses and microbial pollution of air are also covered. Different techniques for measuring air contamination like sedimentation, impaction, and filtration are outlined.