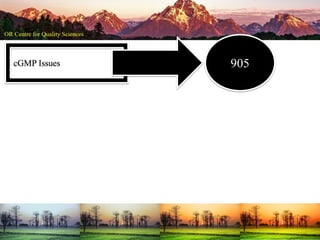
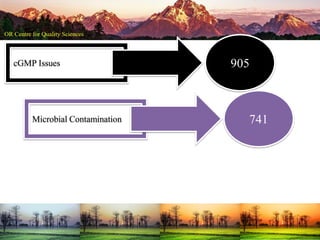
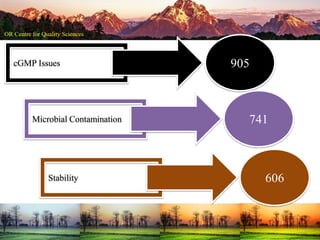
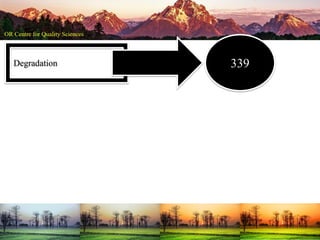
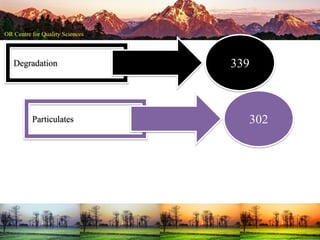
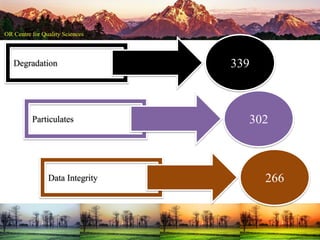
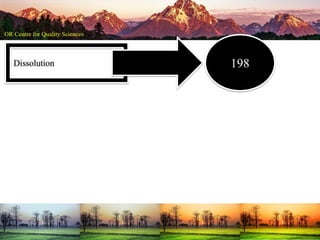
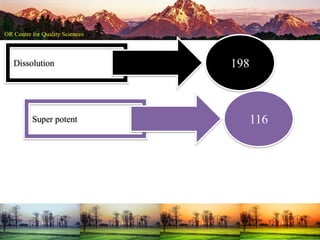
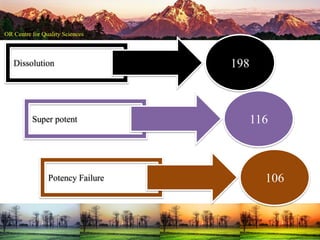
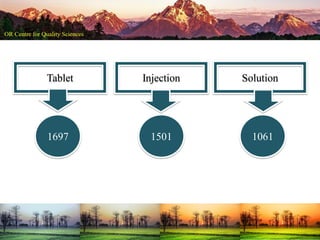
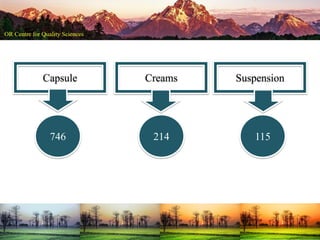
This document summarizes a presentation on pharmaceutical quality given at a PPE conference on August 25, 2020. It notes that the pharmaceutical industry in the country is dependent on imports for knowledge, technology, equipment, and materials. It discusses challenges like quality concerns, import alerts, and knowledge crises. It emphasizes that pharmaceutical quality depends on the quality of development and effectiveness of quality management systems. The presentation argues that to ensure quality, errors of omission and commission must be eliminated through quality by design and standard operating procedures. It concludes that leadership should focus on developing others and facilitating continual improvement rather than just aiming for minimum standards.