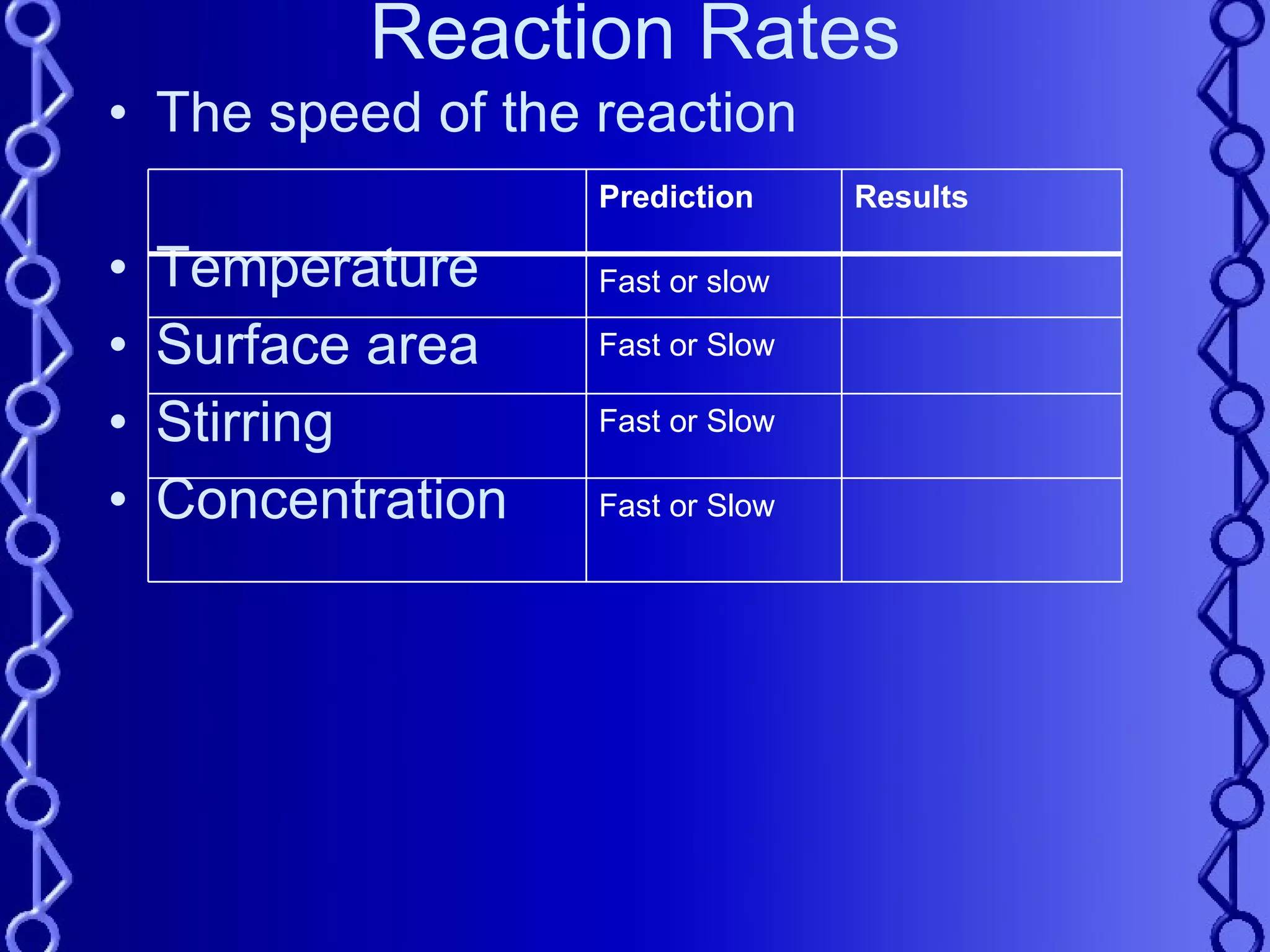
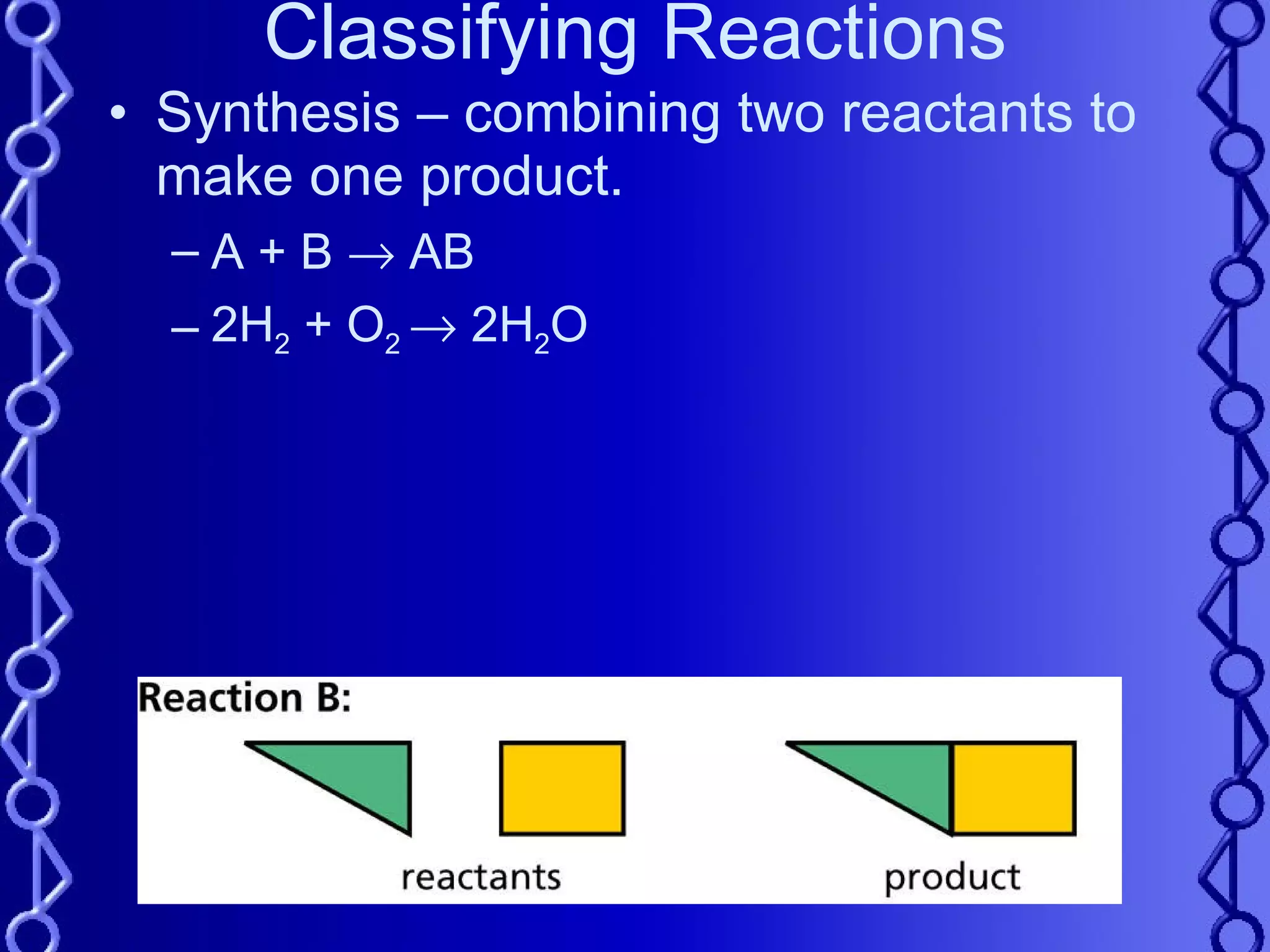
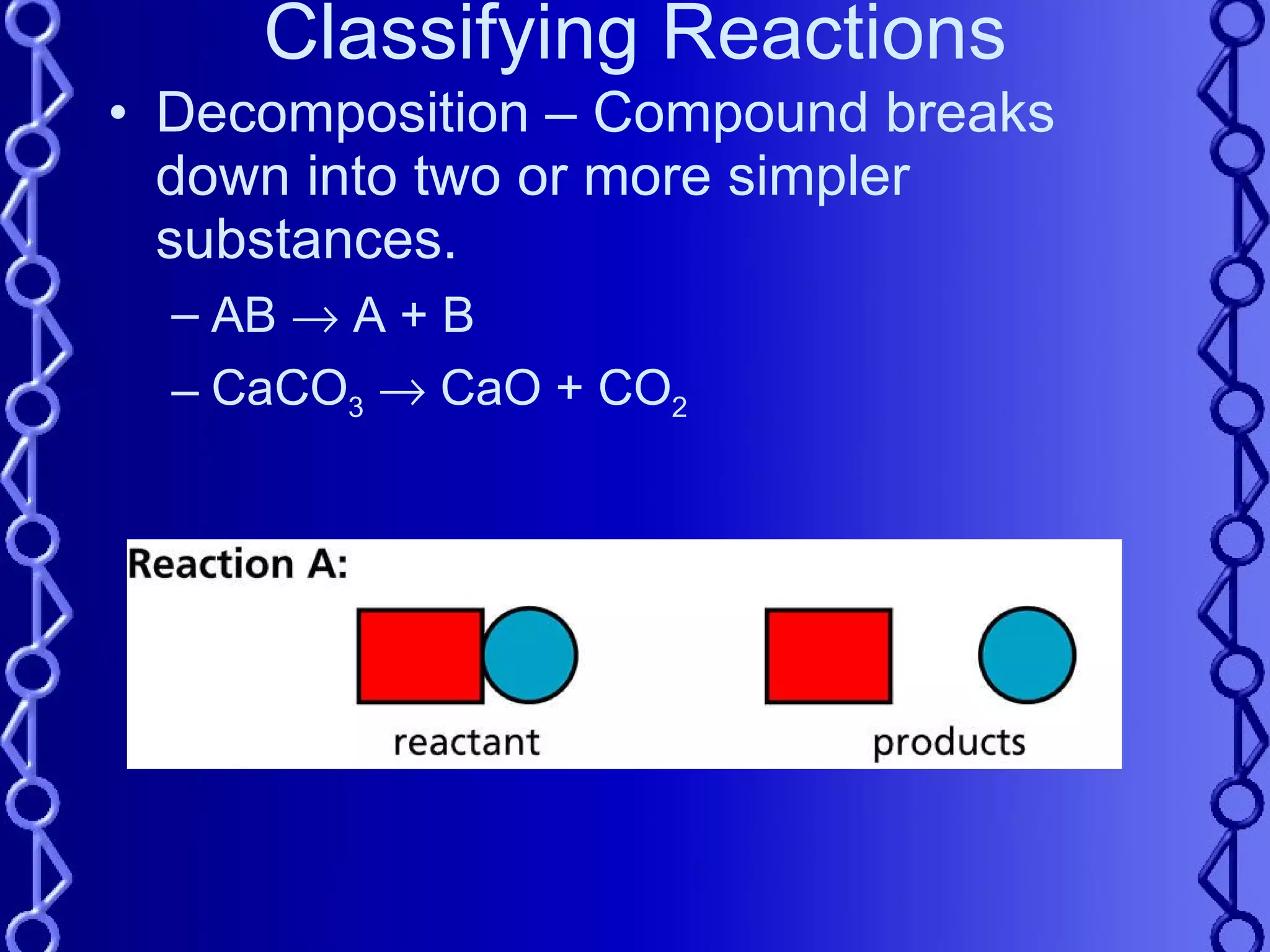
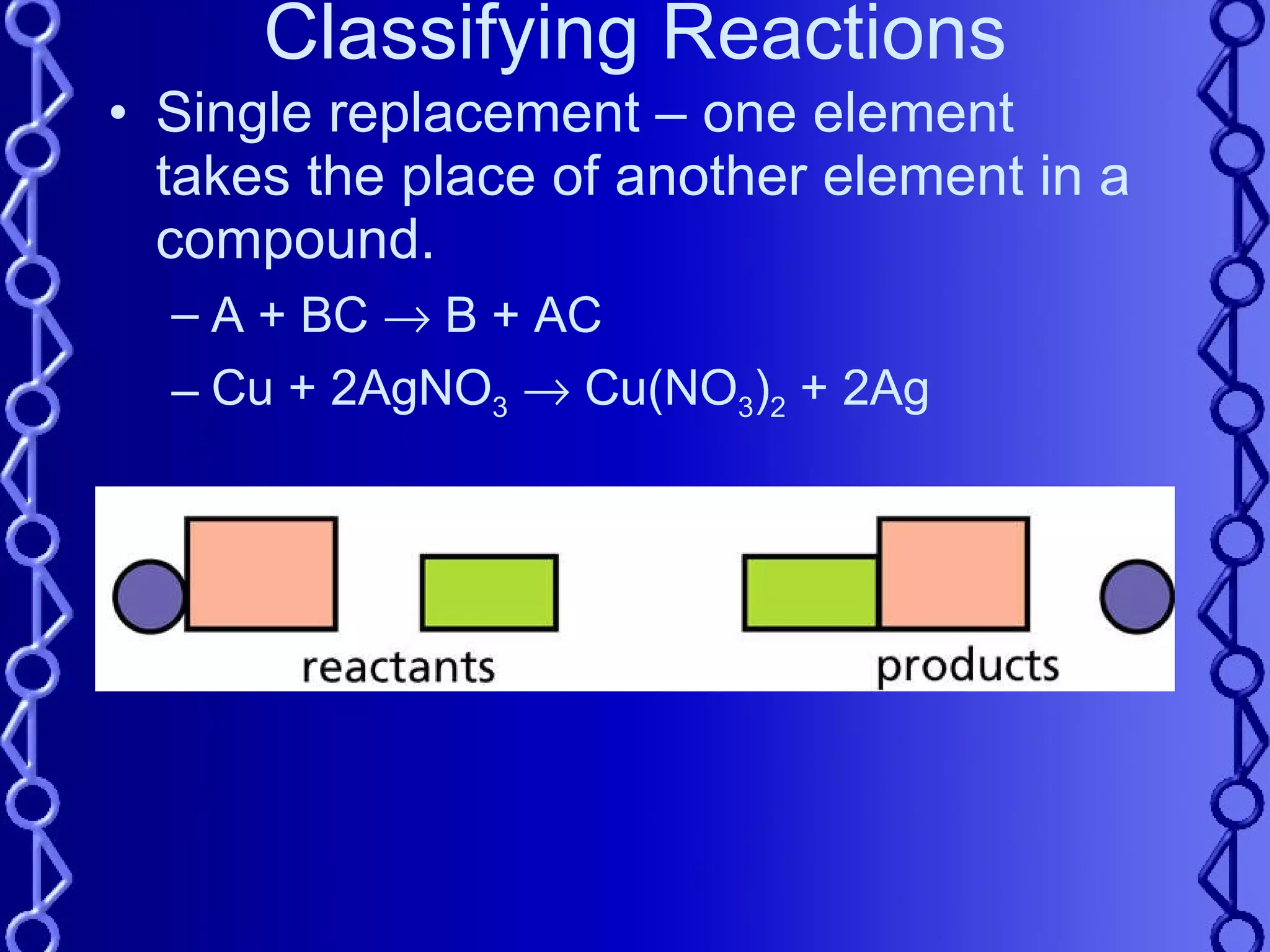
The document discusses types of chemical reactions and factors that affect reaction rates. It defines five types of reactions:
1) Synthesis reactions combine two reactants to form one product.
2) Decomposition reactions involve a compound breaking down into simpler substances.
3) Single replacement reactions occur when one element replaces another in a compound.
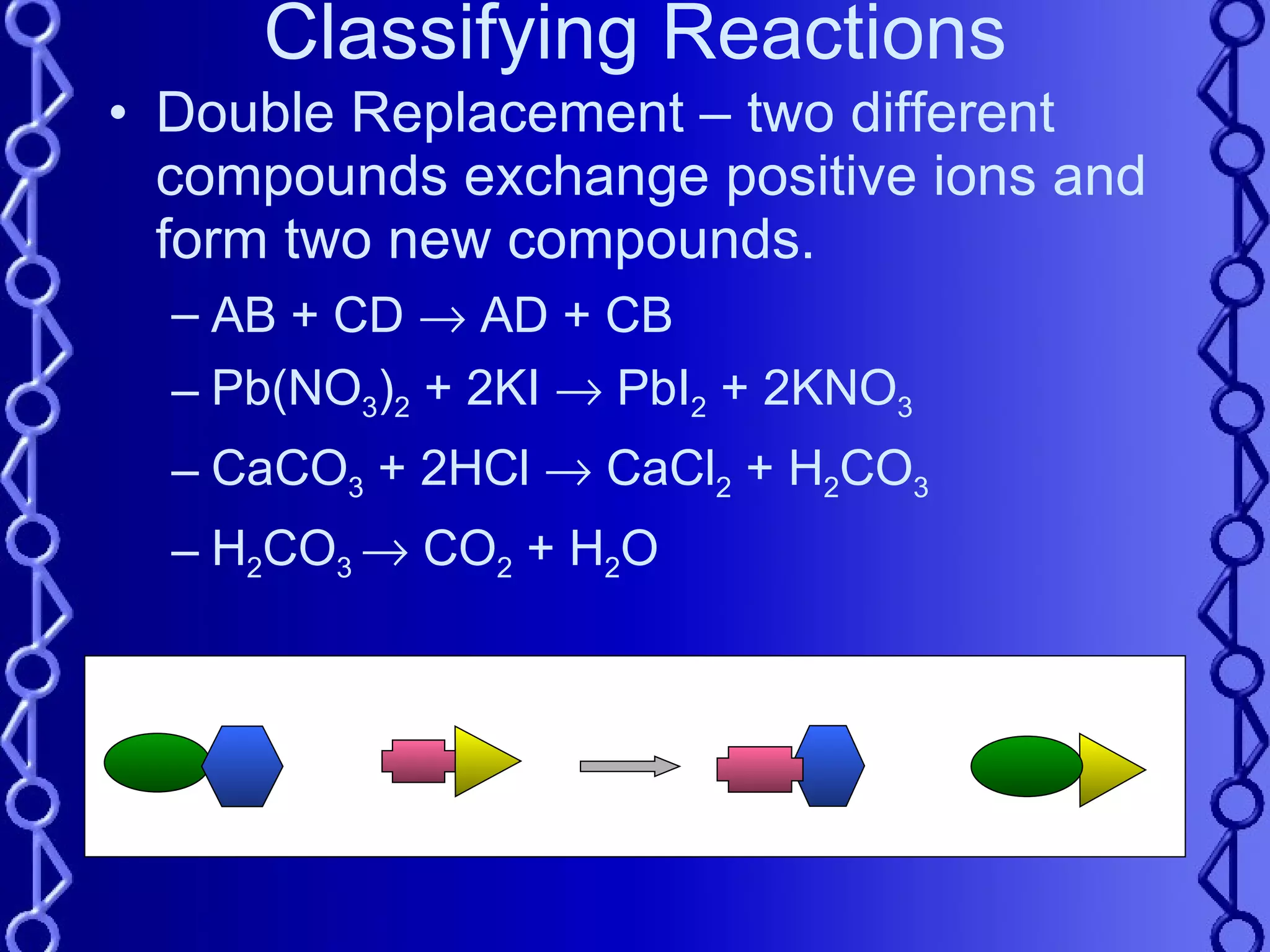
4) Double replacement reactions involve two compounds exchanging ions to form two new compounds.
5) Combustion reactions involve rapid reaction with oxygen, often producing heat and light.