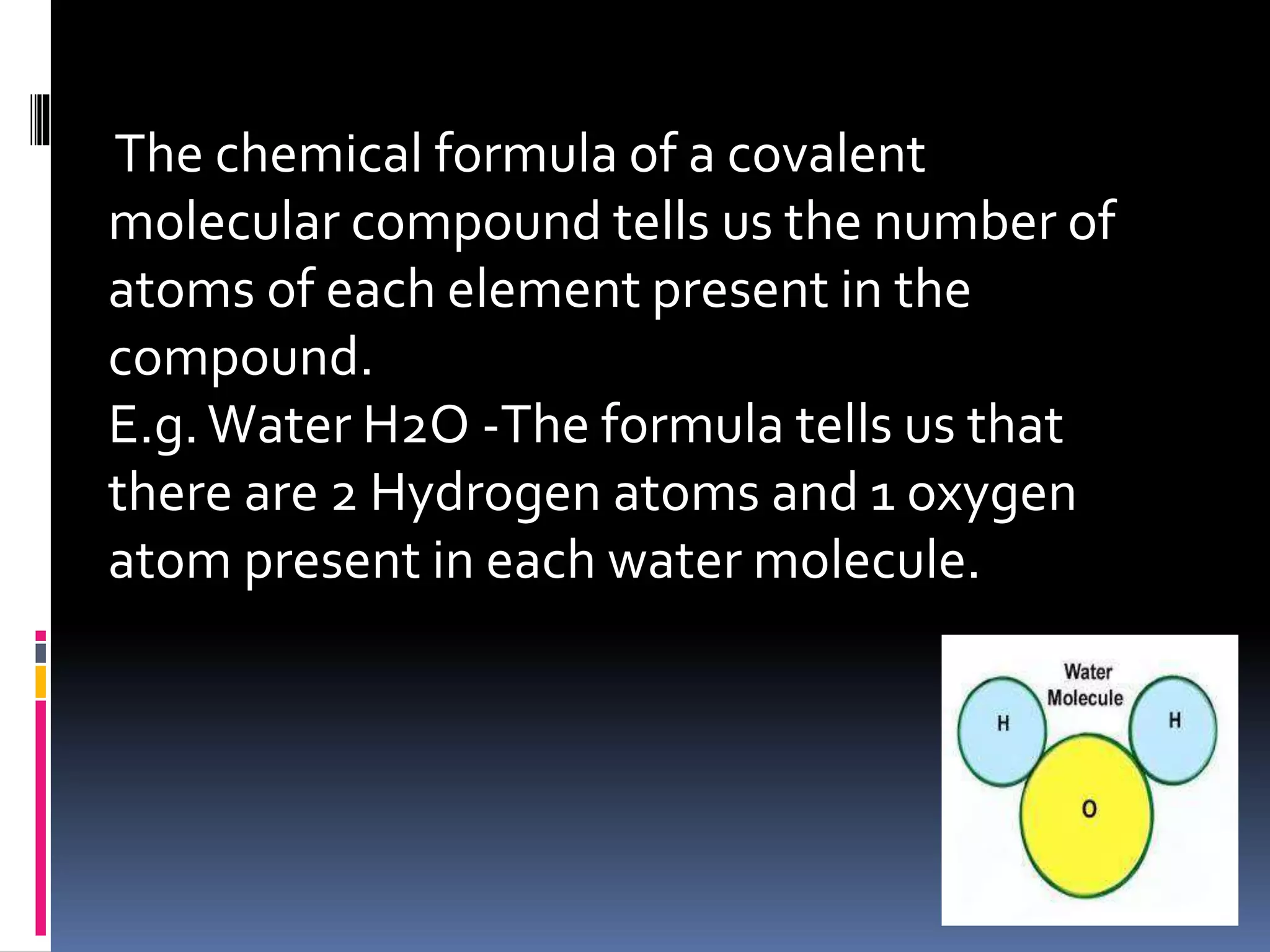
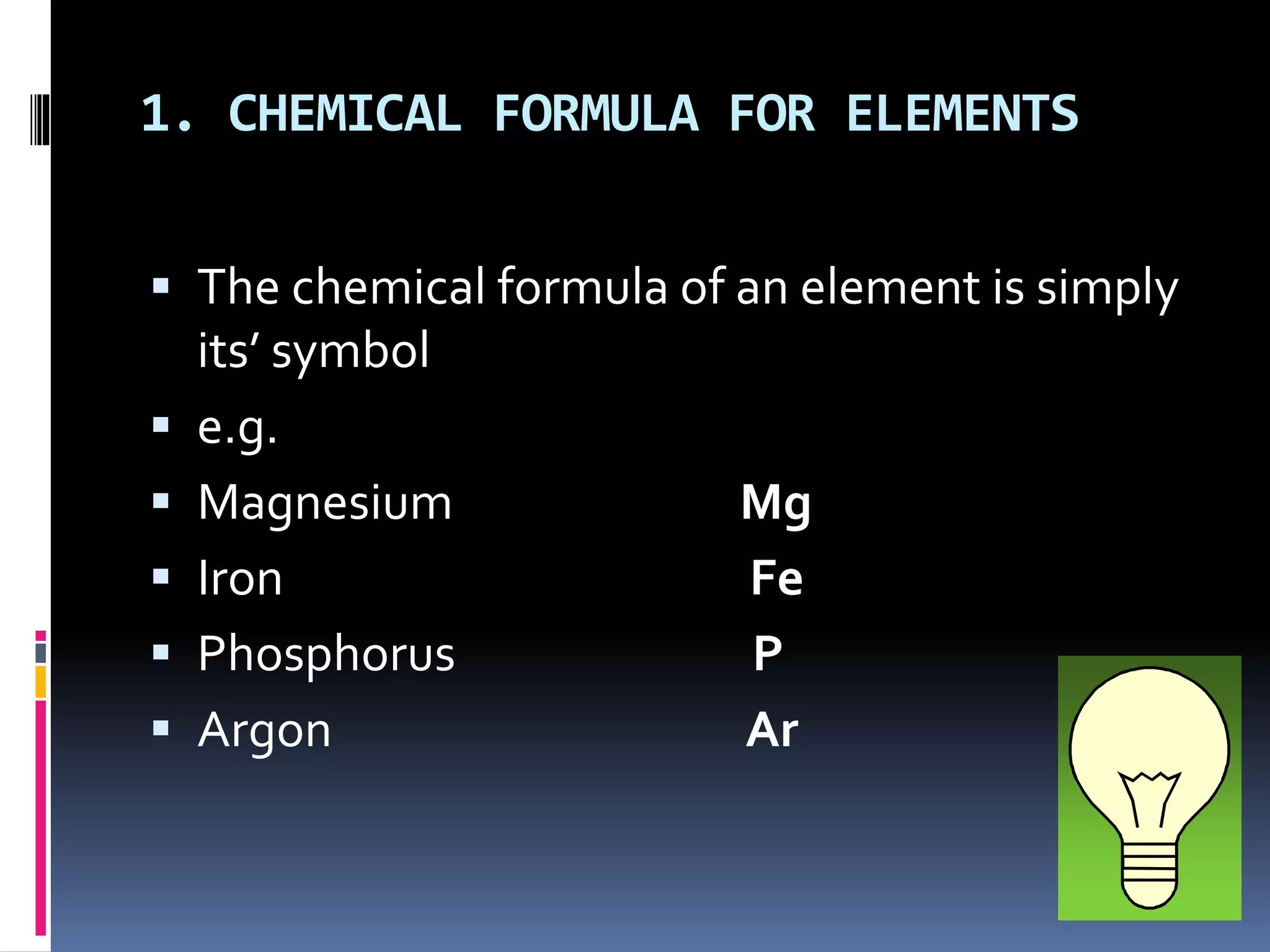
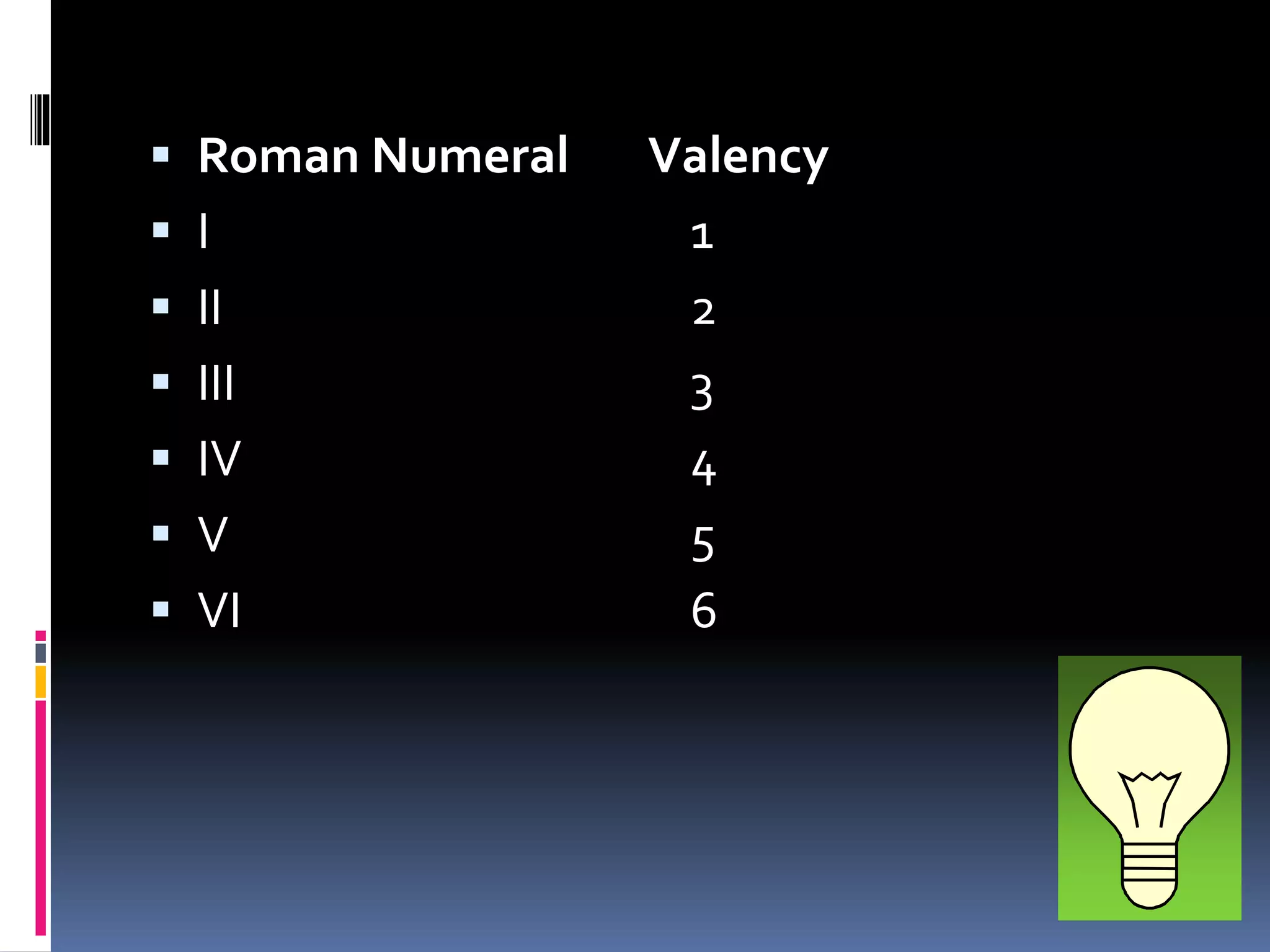
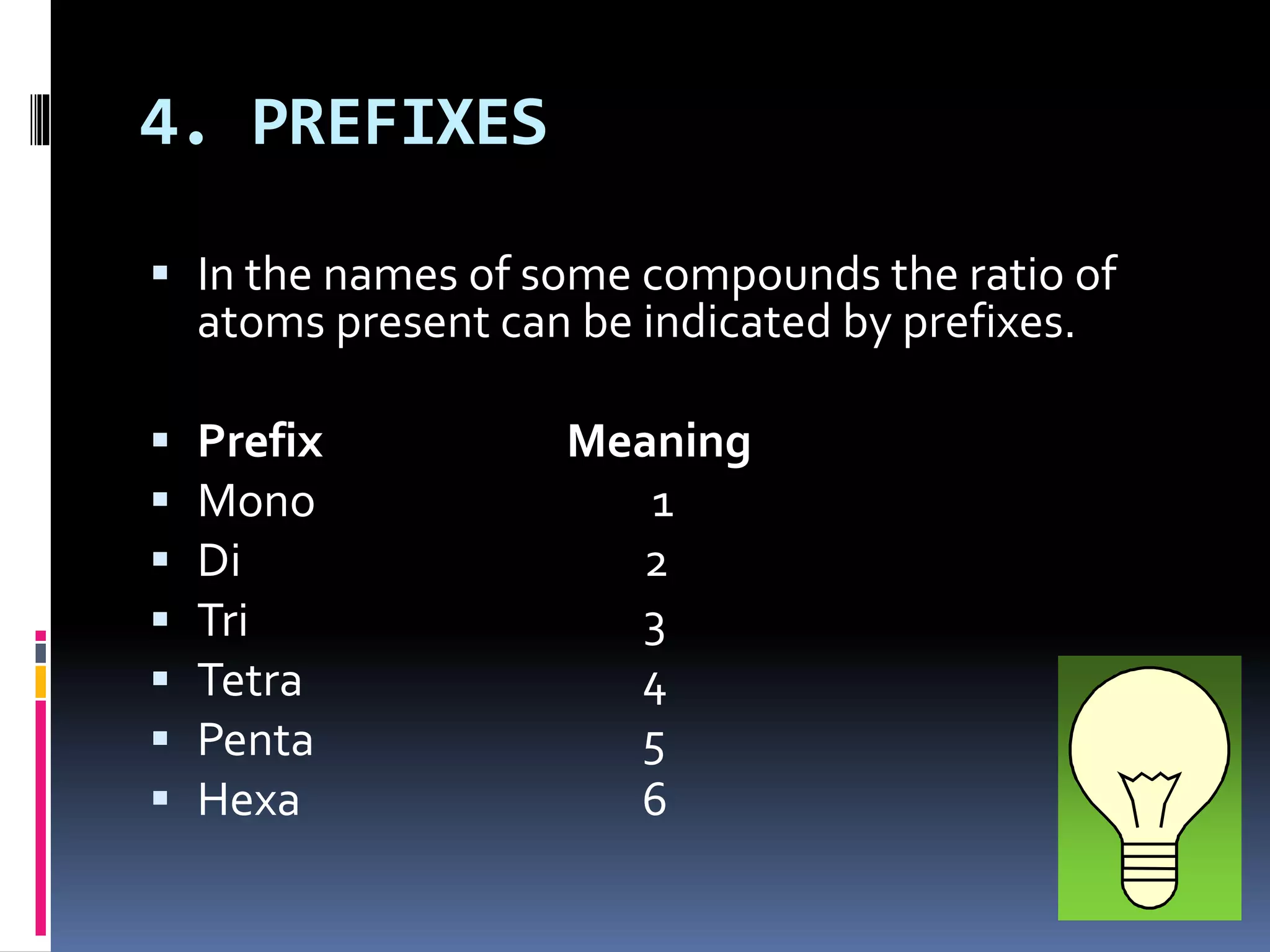
The document discusses chemical formulas and how they are used to express information about the proportions of atoms in compounds. It covers:
- Chemical formulas for covalent molecular compounds, covalent networks, and ionic compounds show the number or ratio of elements present
- Formulas are written using element symbols and subscripts to indicate atom counts
- Valence and Roman numerals are used to indicate an element's bonding tendency which helps write formulas
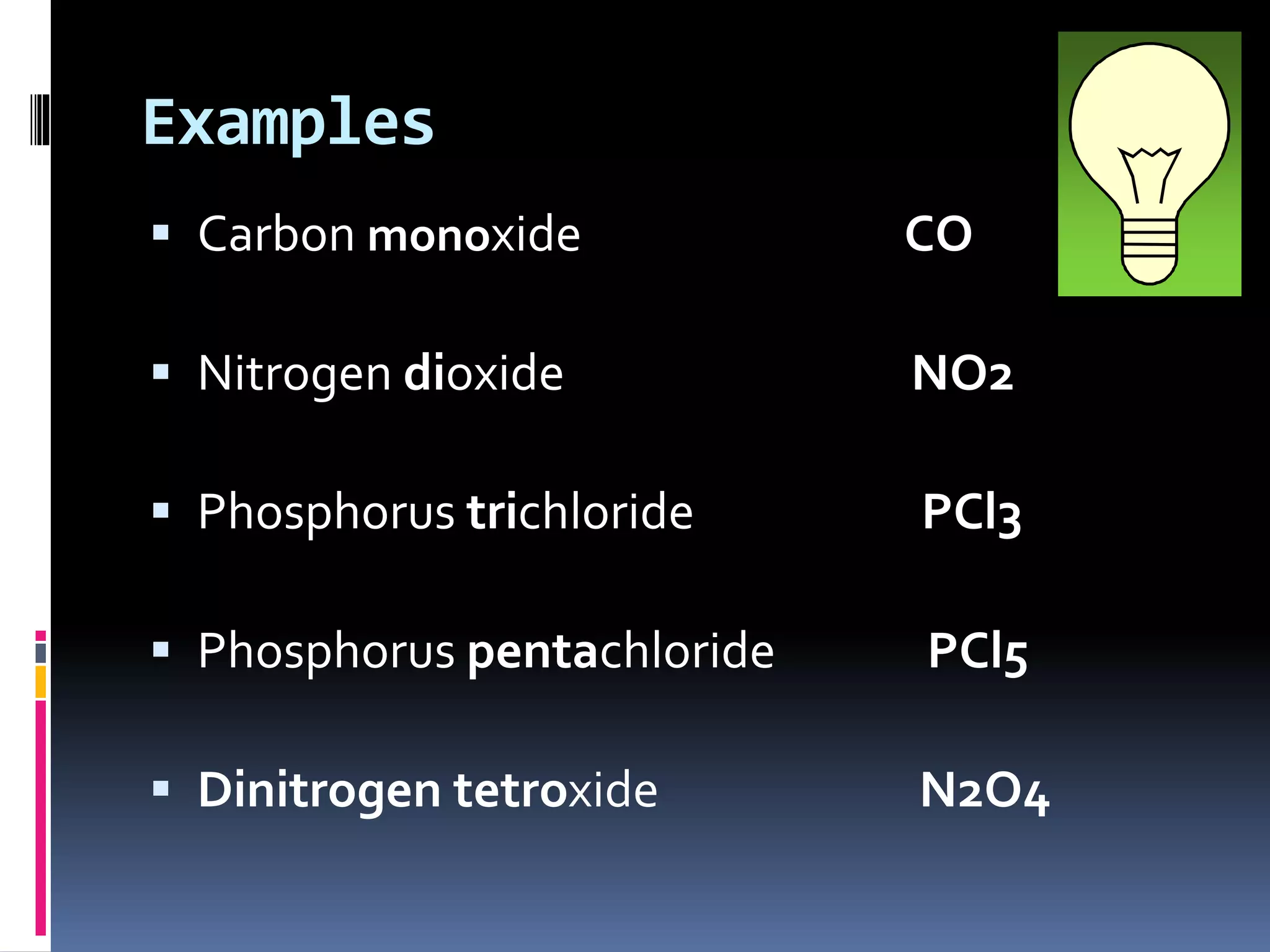
- Prefixes like mono-, di-, tri- are also used to show atom ratios
- Polyatomic ions, ions formed of multiple elements, are also represented in formulas
- Ionic formulas show the charges of ions present in ionic substances