The document describes the major historical atomic theories:
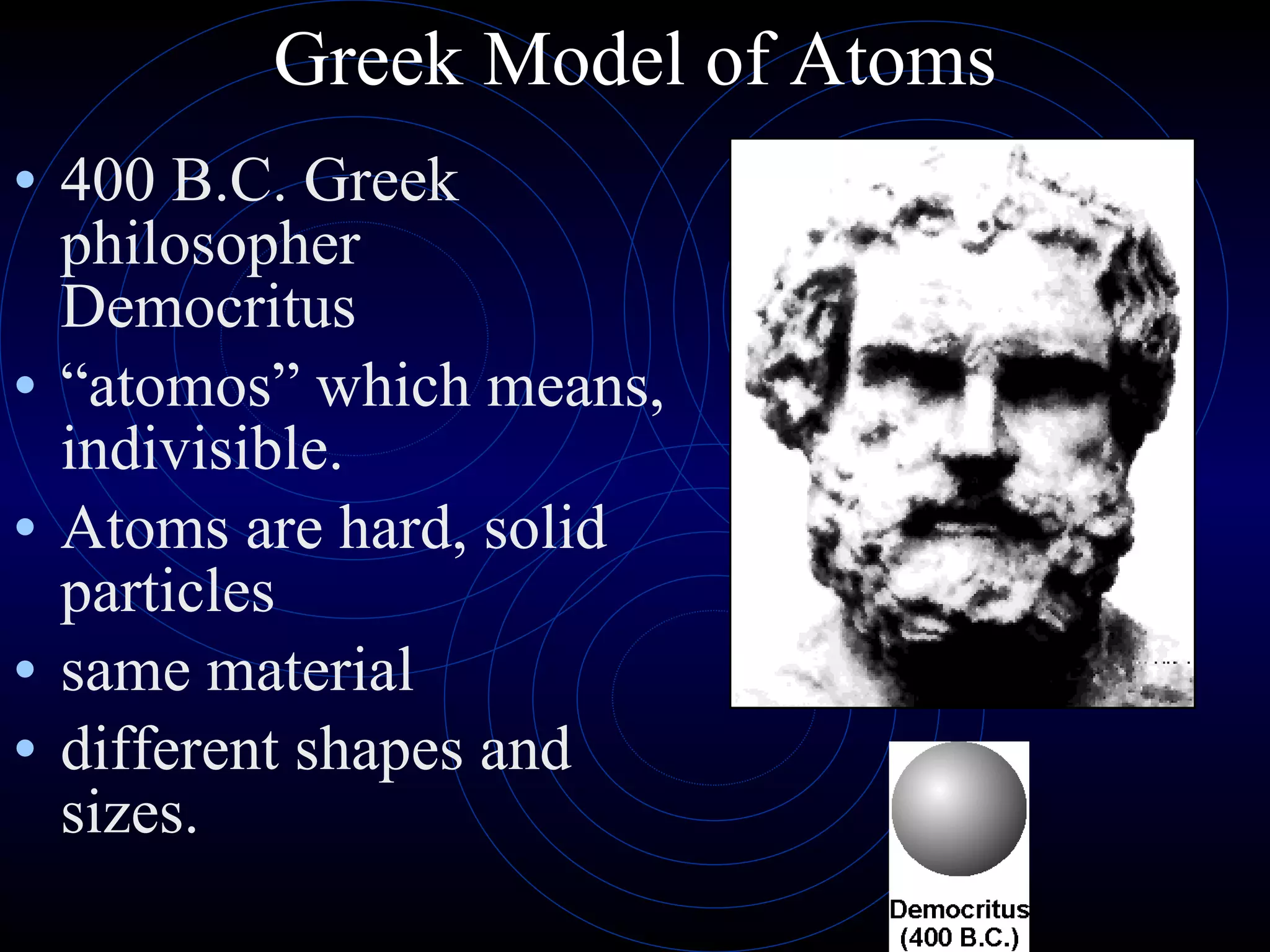
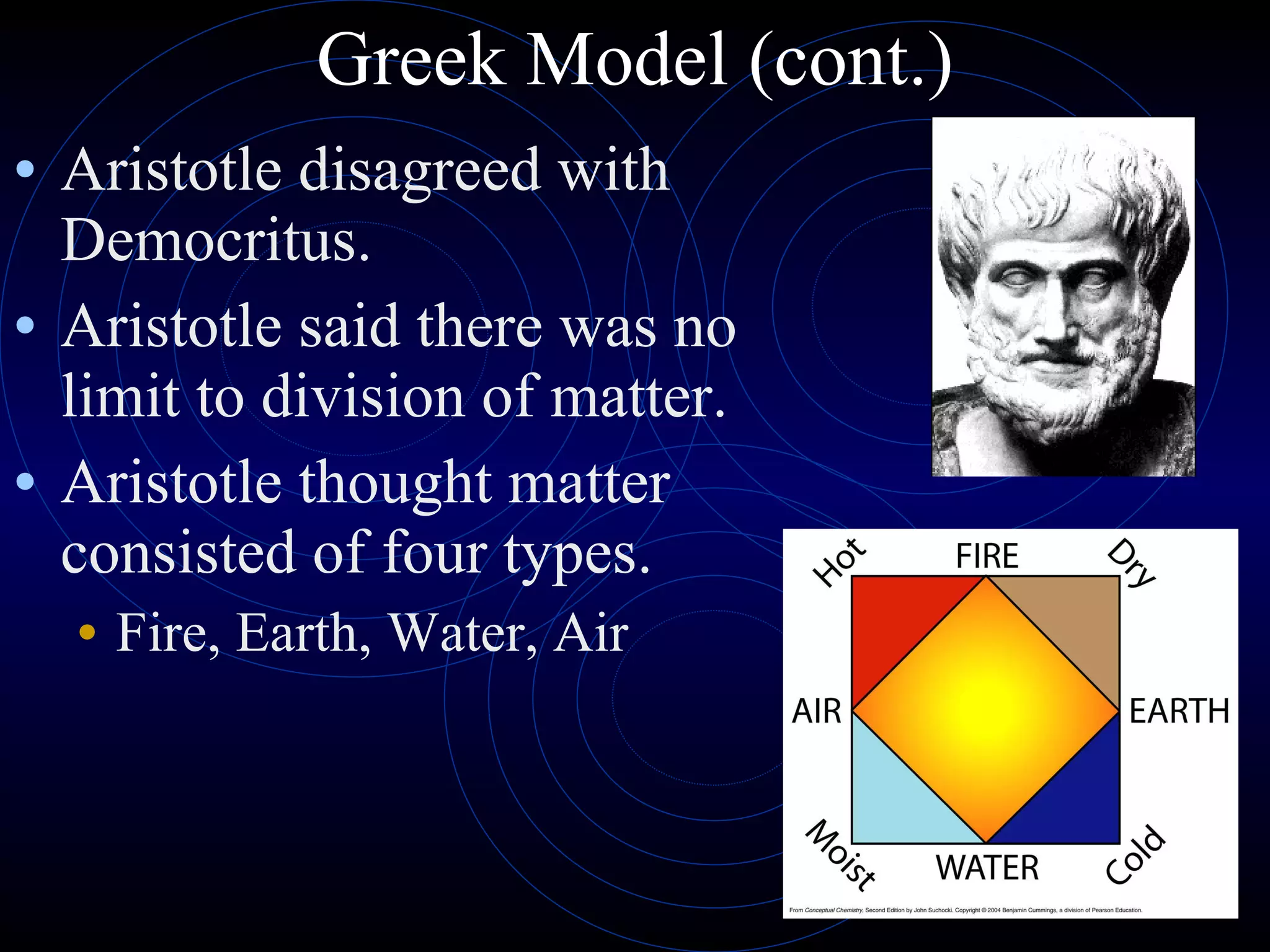
1) The Greek model proposed by Democritus that all matter is made of indivisible atoms of different shapes and sizes. Aristotle disagreed and proposed matter is made of four elements.
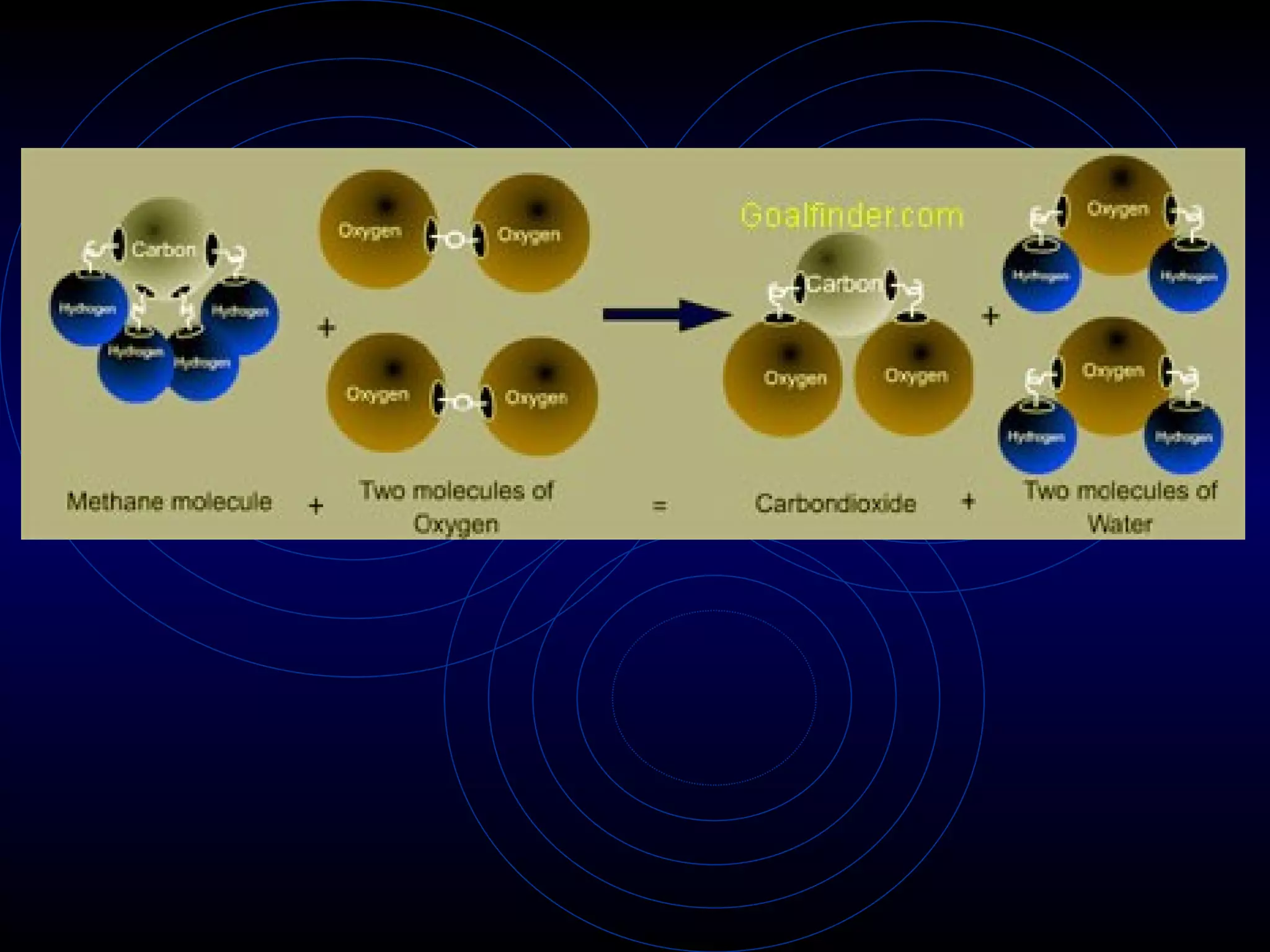
2) Dalton's atomic theory (1803) proposed that elements are made of atoms, atoms of the same element have the same mass, and compounds contain atoms of different elements combined in fixed ratios.
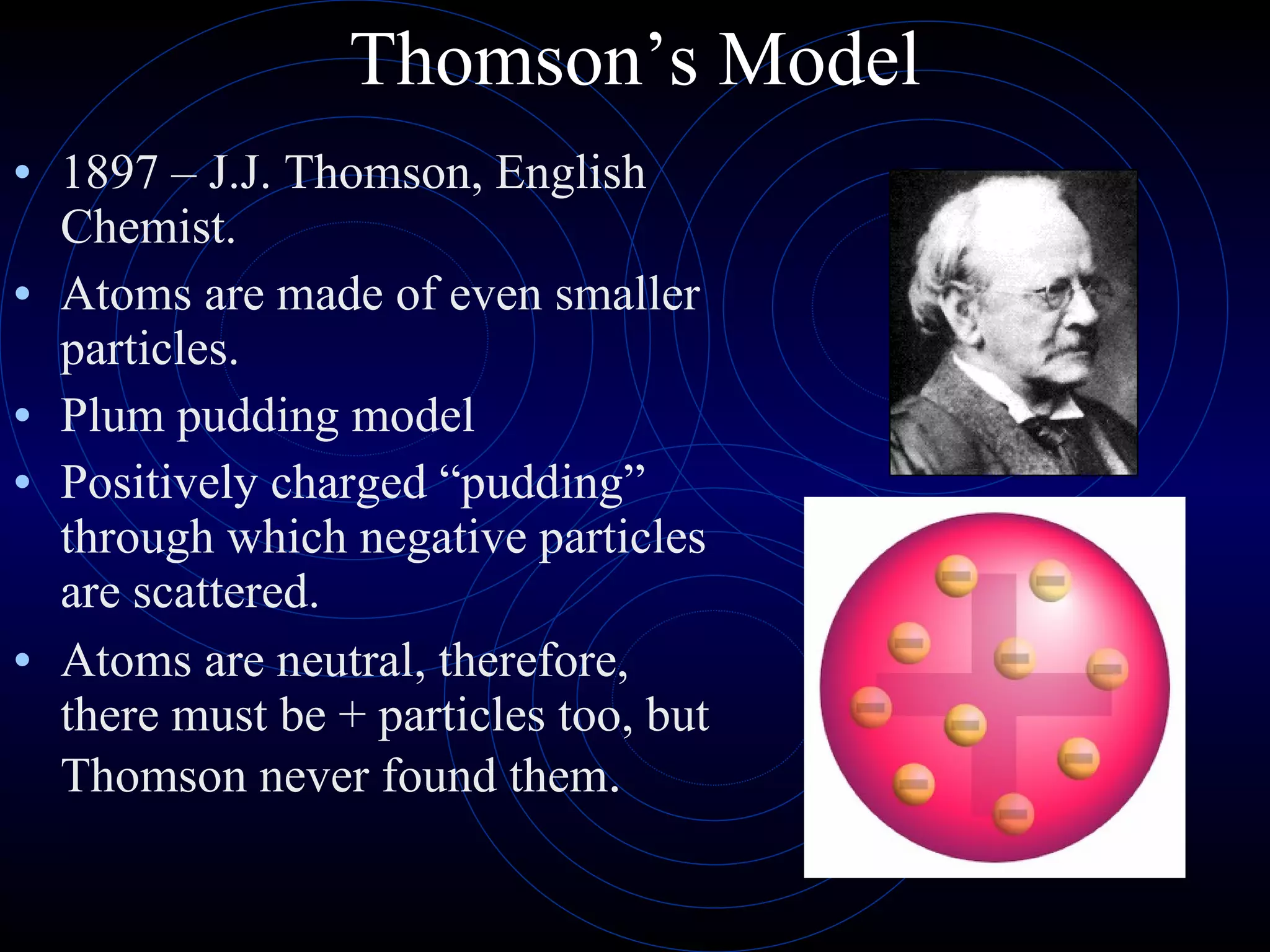
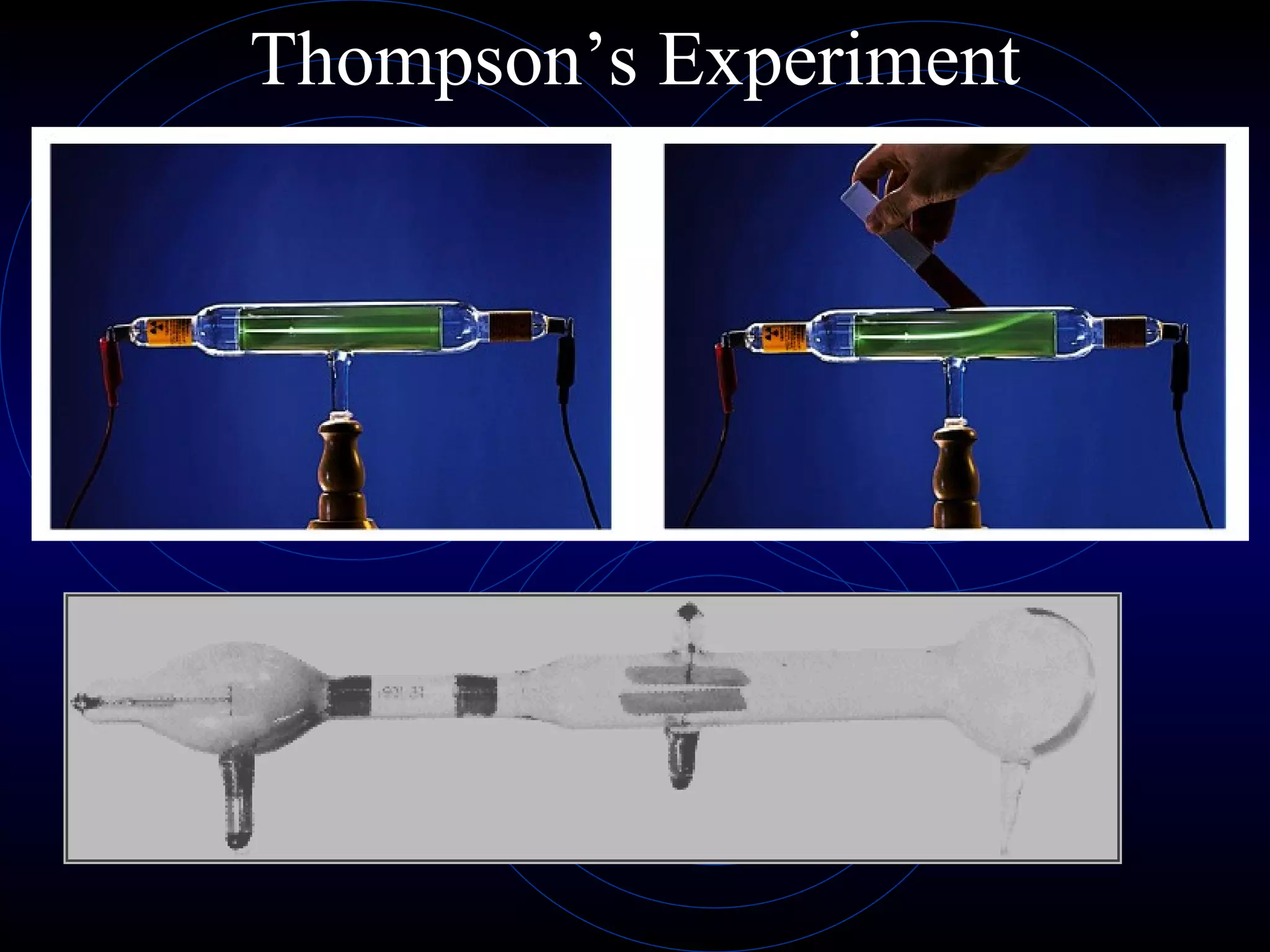
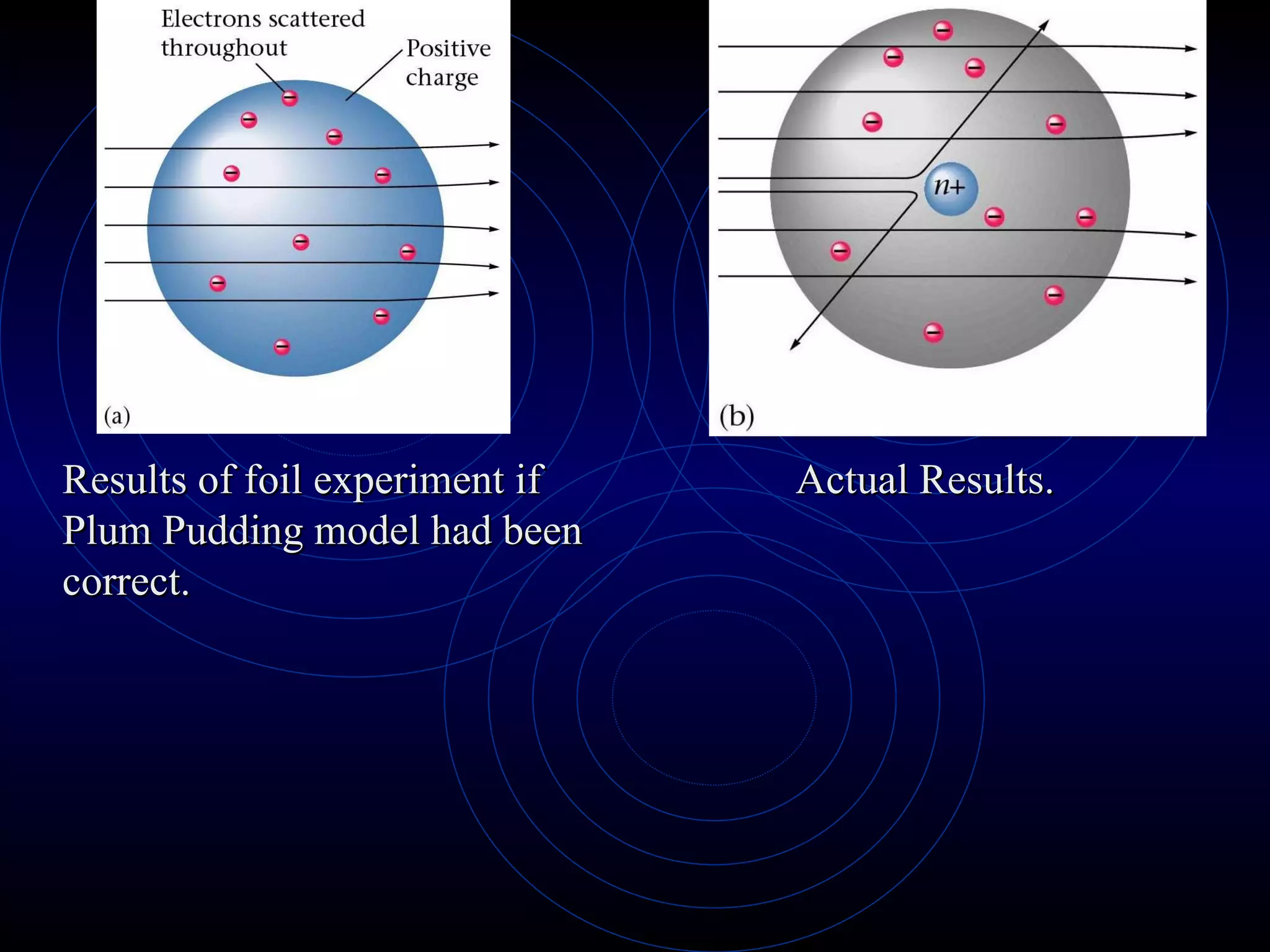
3) Thomson's model (1897) proposed atoms are made of even smaller particles in a "plum pudding" model, with positive charge throughout and negative electrons scattered within.
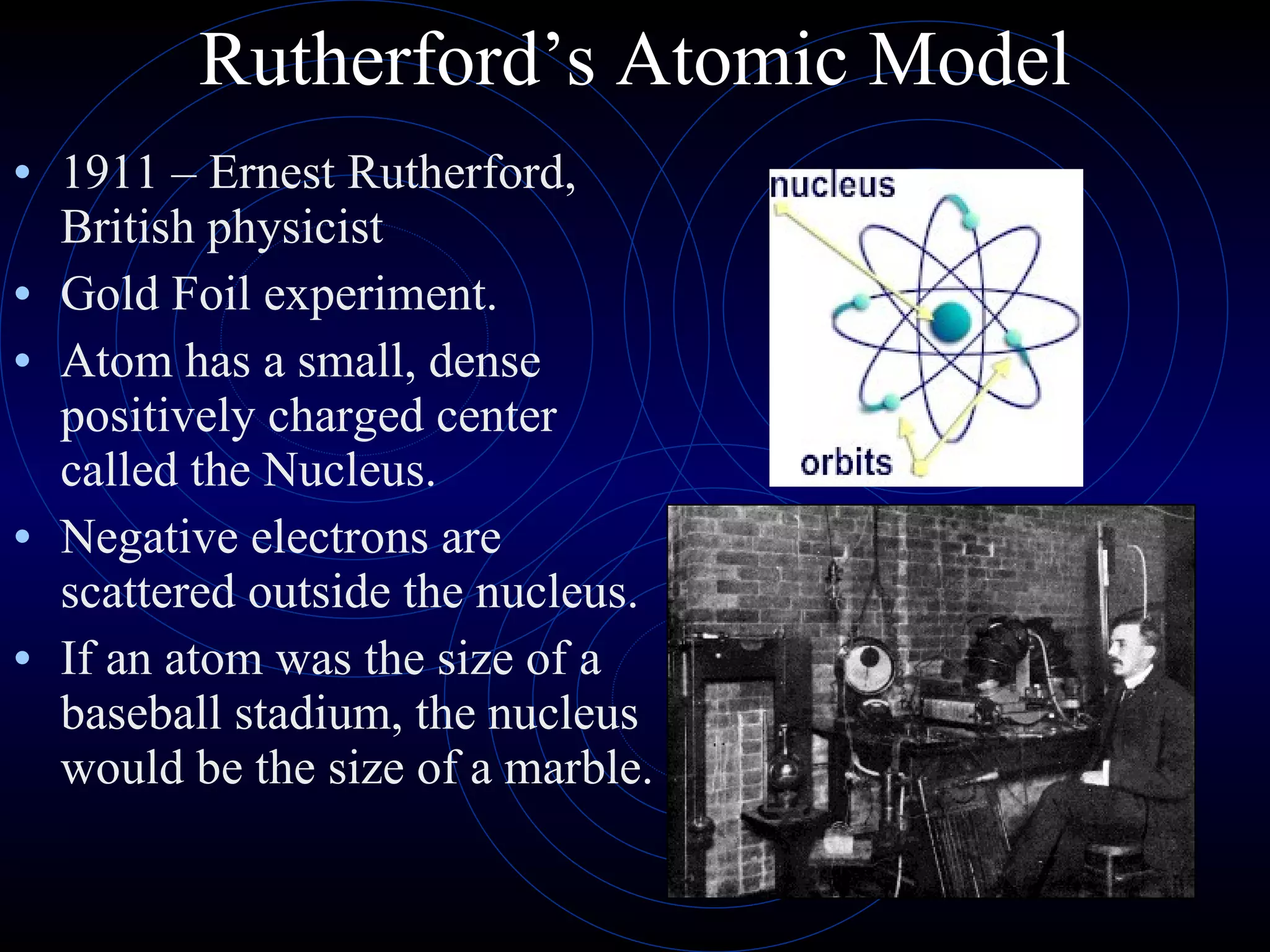
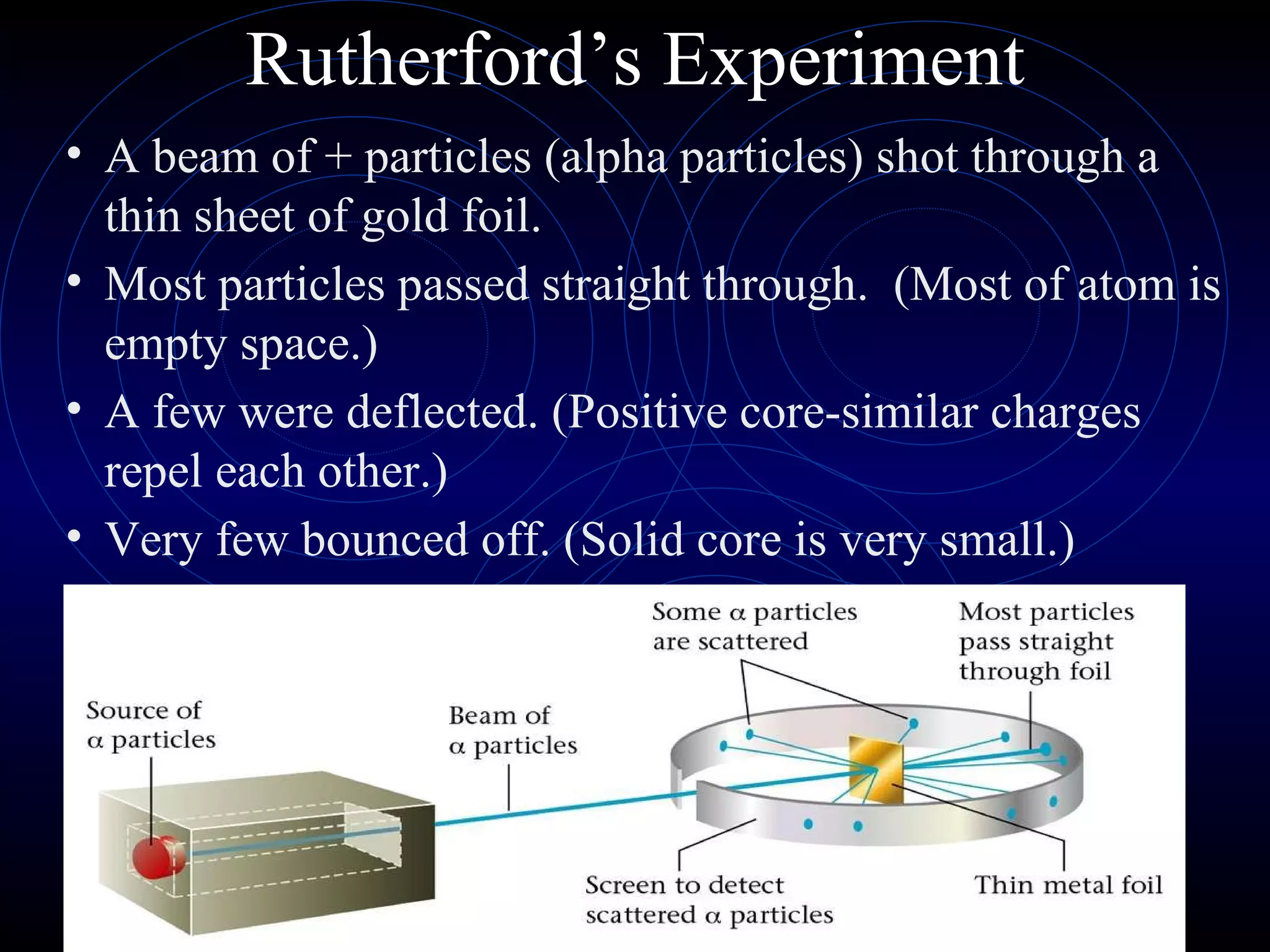
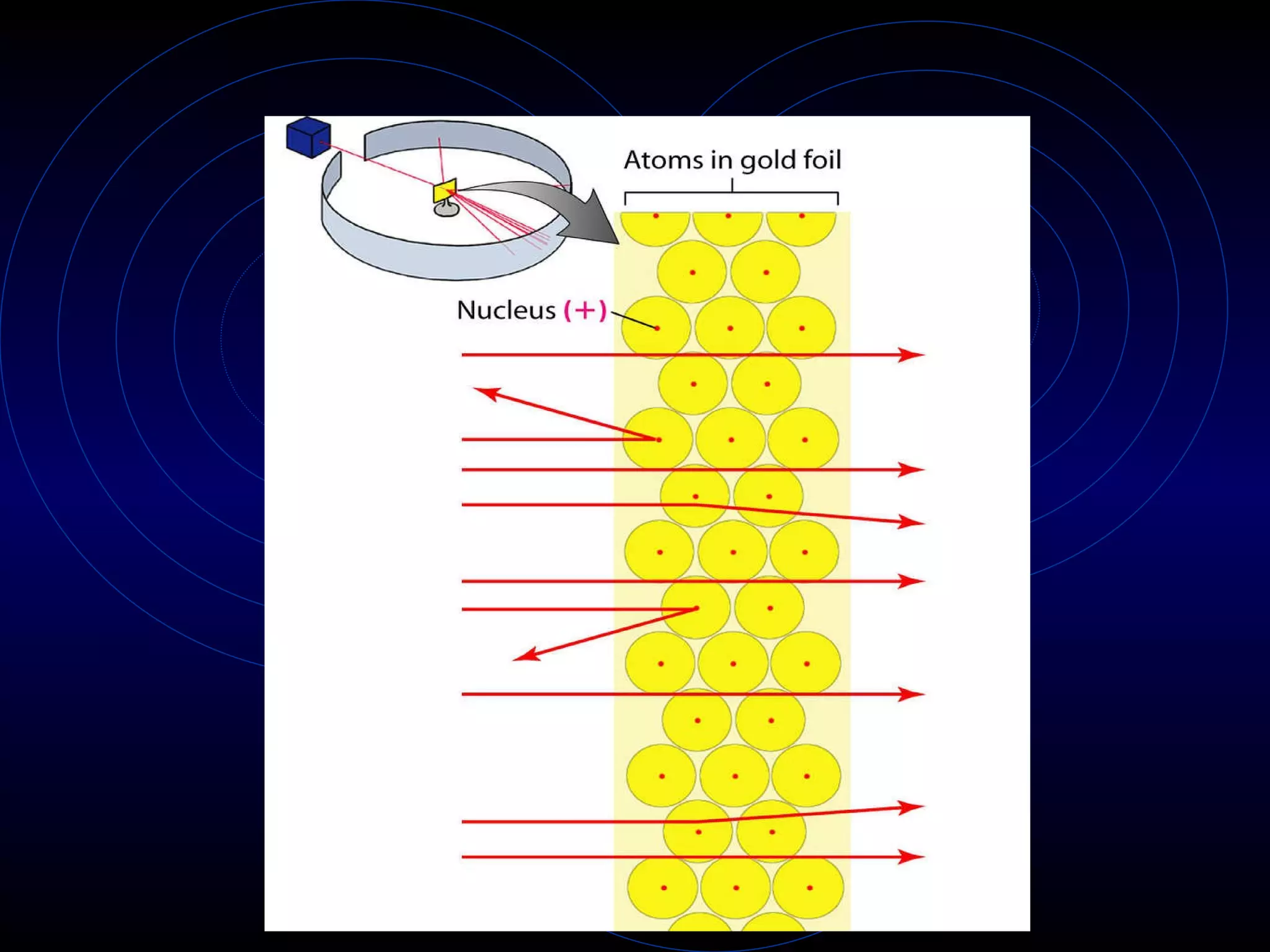
4) Rutherford's model (1911) from his gold foil experiment showed atoms have a