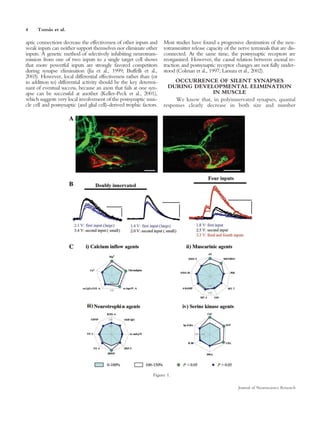
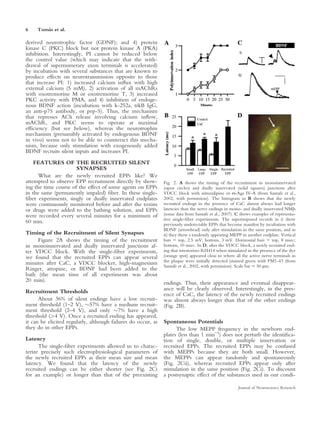
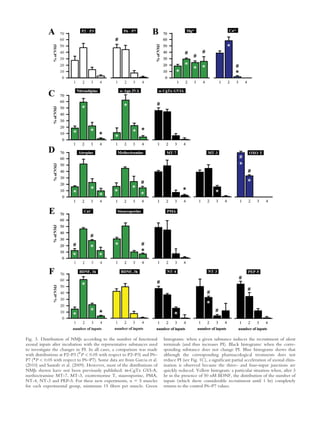
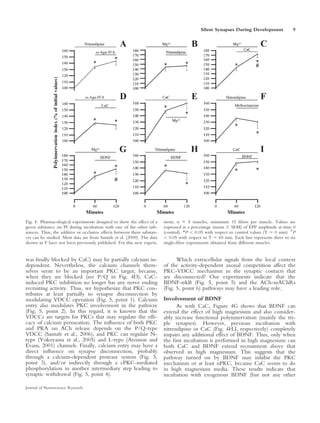
This document summarizes evidence that some synaptic contacts become "silent" during postnatal synapse elimination in muscle, retaining the ability to release acetylcholine. The authors investigated whether blocking certain molecular pathways could induce the functional recruitment of these silent synapses. They found that blocking muscarinic acetylcholine autoreceptors, calcium channels, or protein kinase C, or applying brain-derived neurotrophic factor, increased the number of functional inputs per neuromuscular junction. This suggests silent synapses may be recruited through these molecular mechanisms before being eliminated. The balance between trkB and muscarinic signaling pathways may regulate synaptic suppression during development.