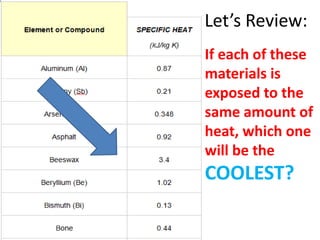
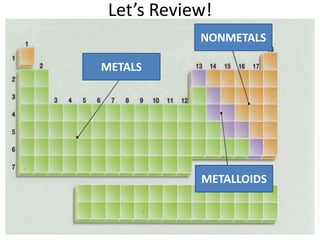
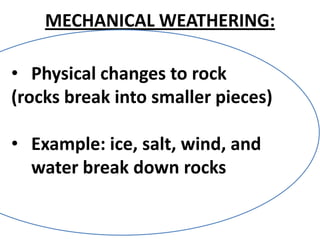
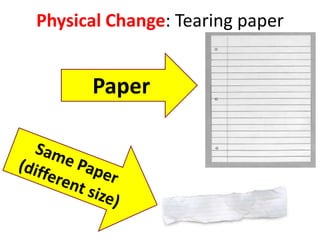
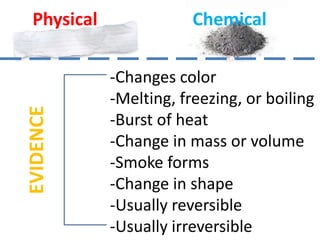
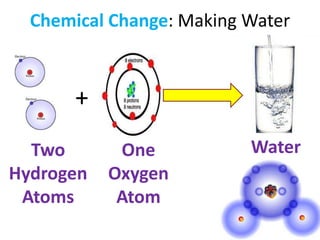
A physical change alters a substance's physical properties but not its chemical identity, while a chemical change transforms a substance into different substances through breaking or forming of chemical bonds. Changes in color, mass, or release of gases indicate a chemical change has occurred, whereas changes in state or phase without a new substance forming signal a physical change. Students can identify if a change is physical or chemical based on observable evidence like changes in properties or formation of new substances.