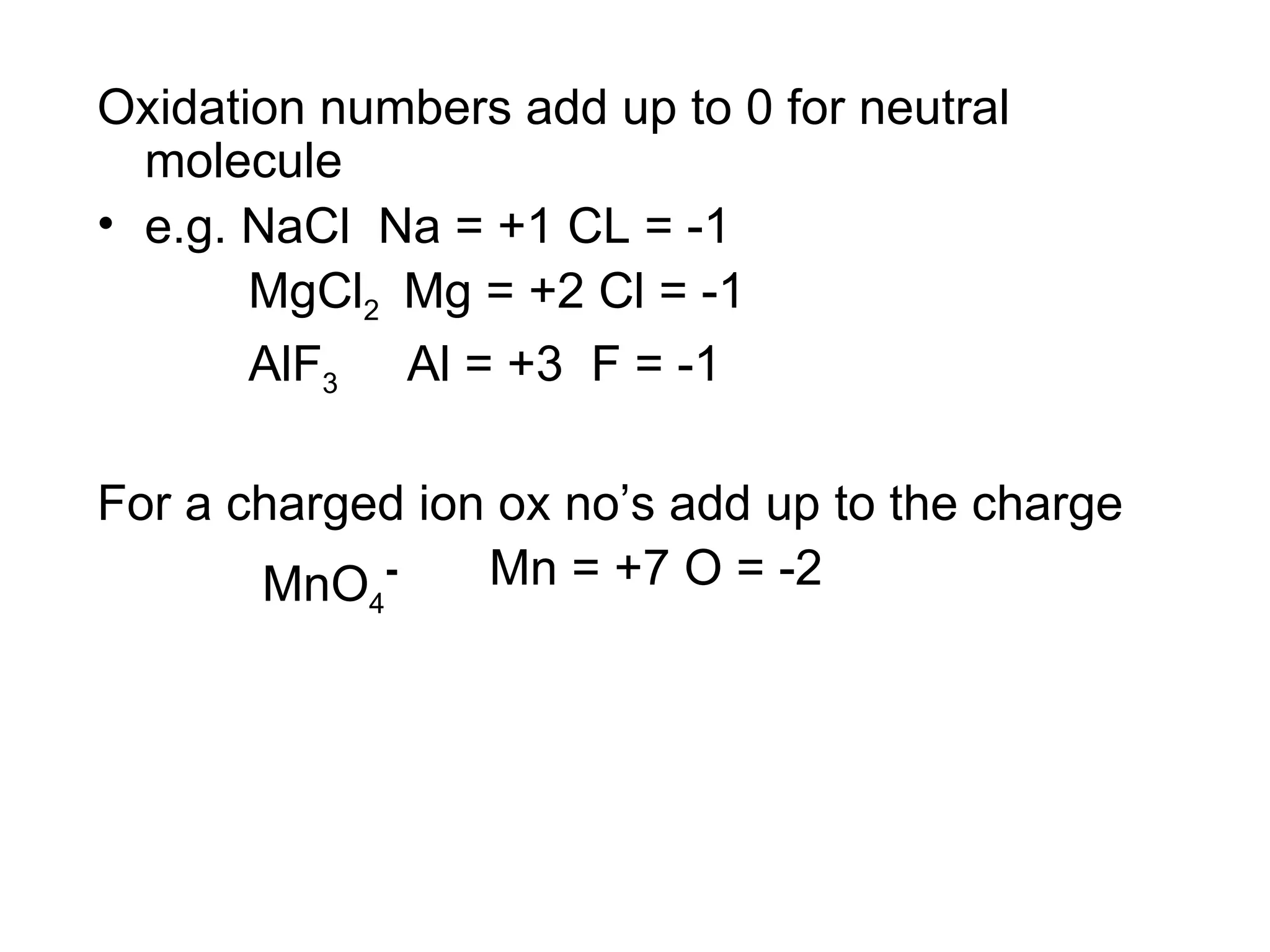
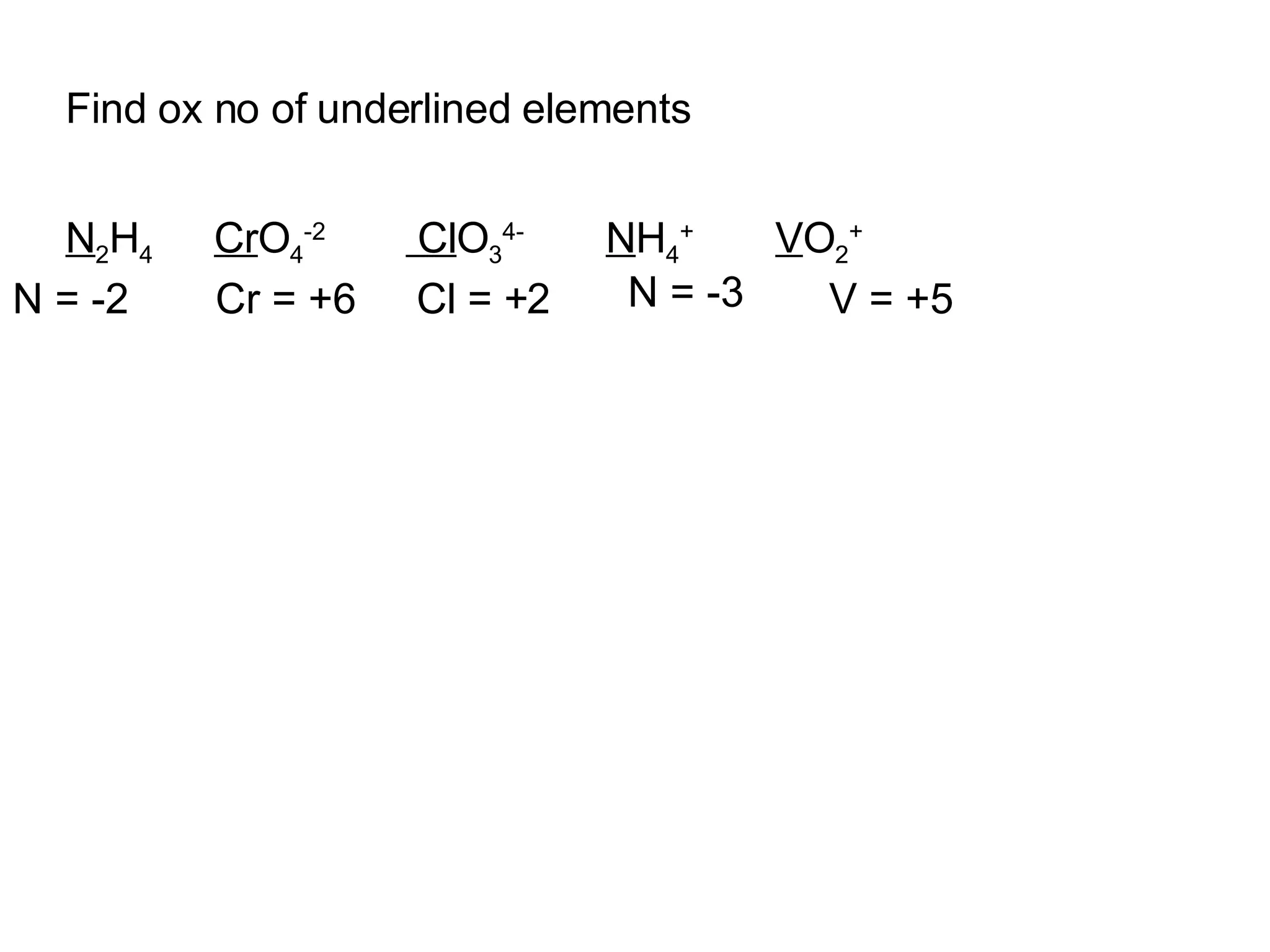
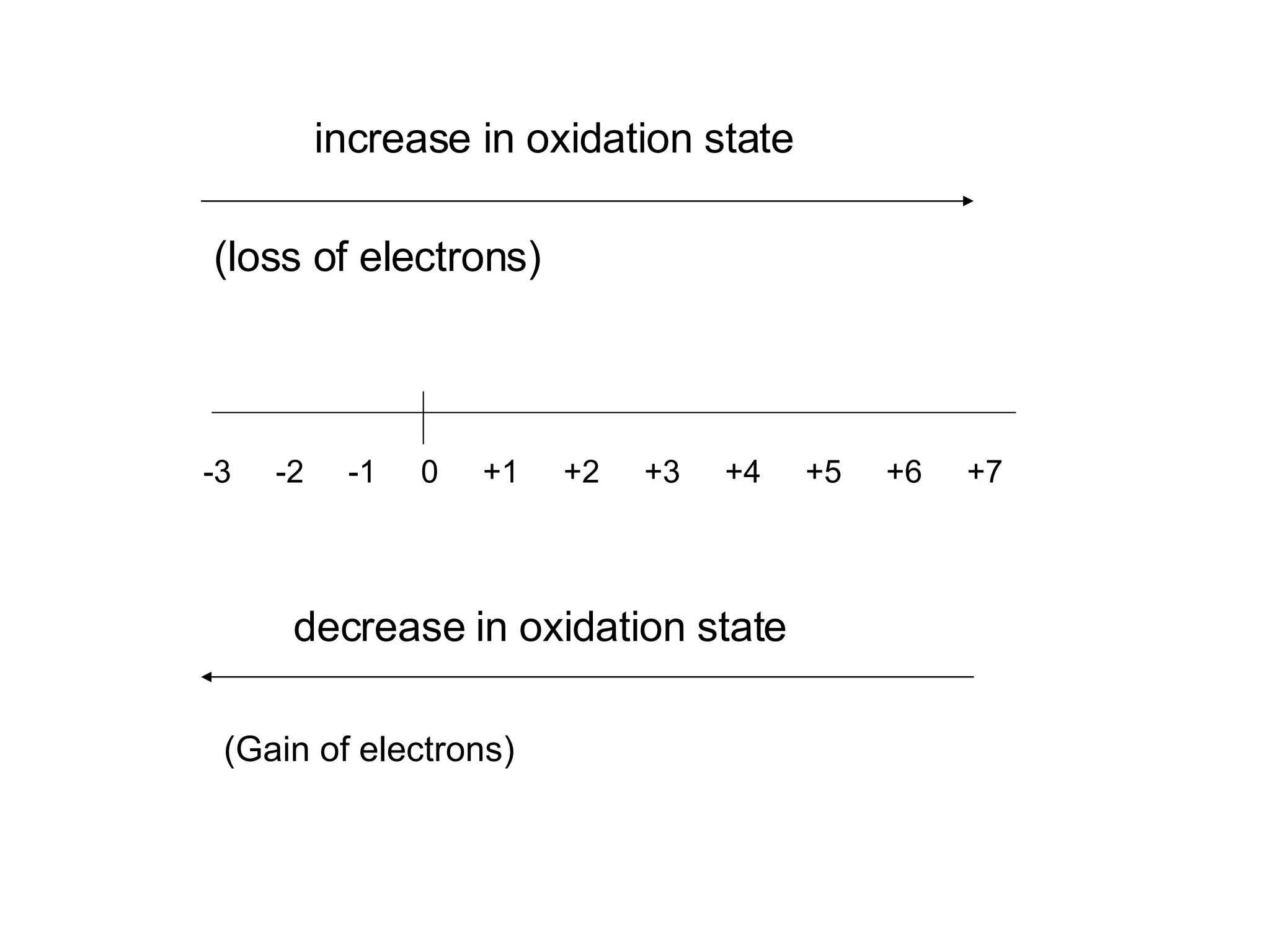
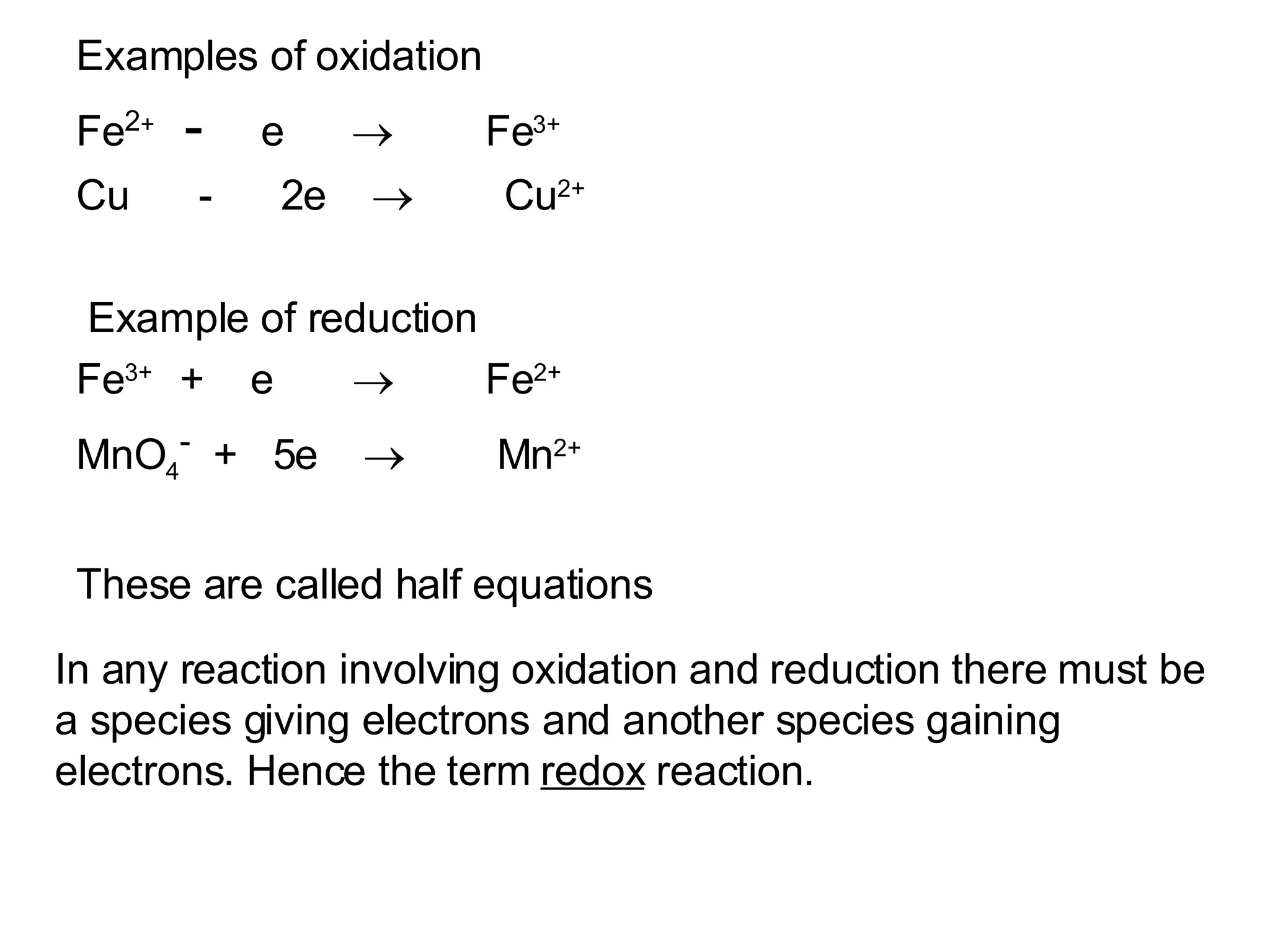
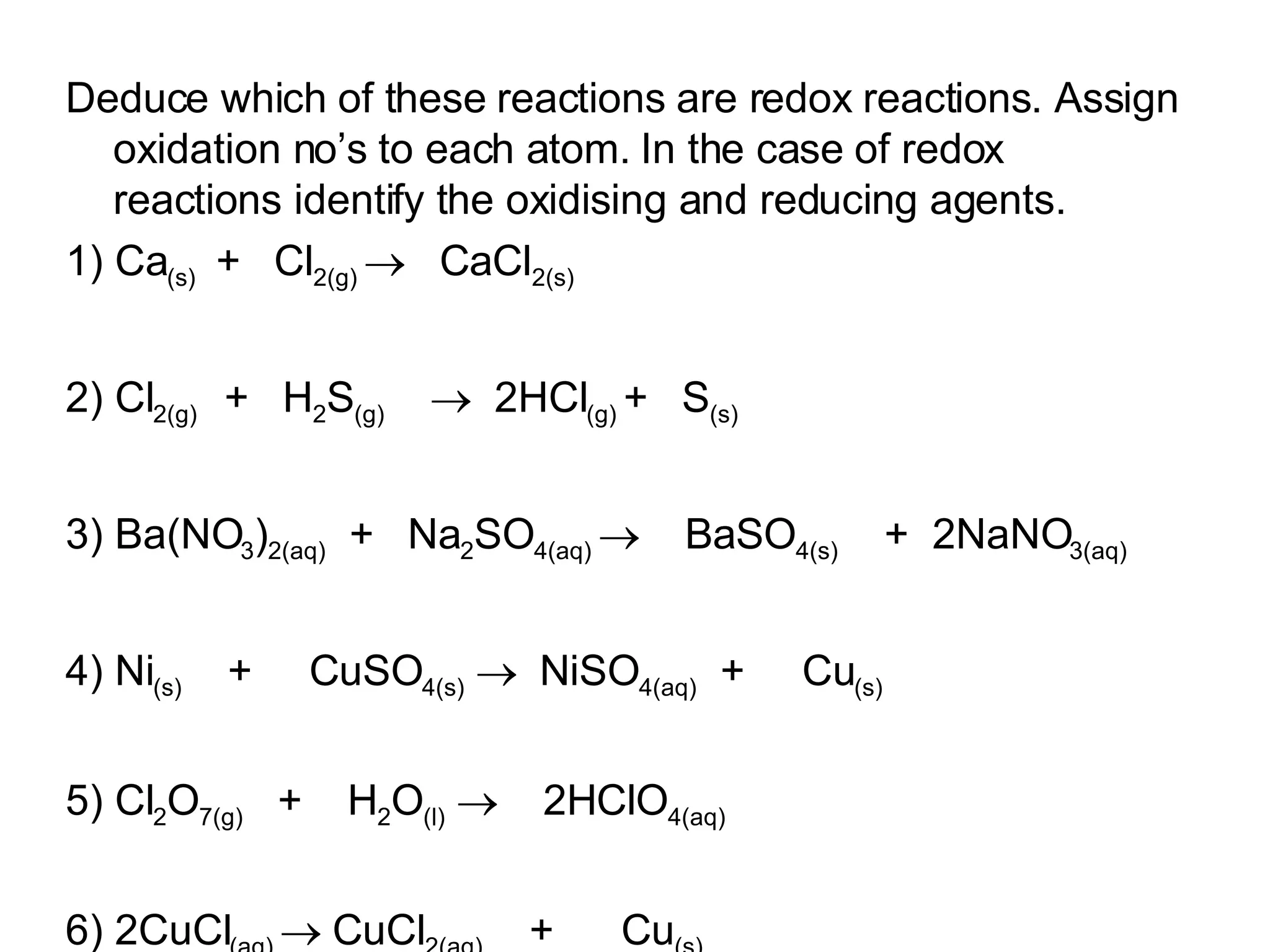
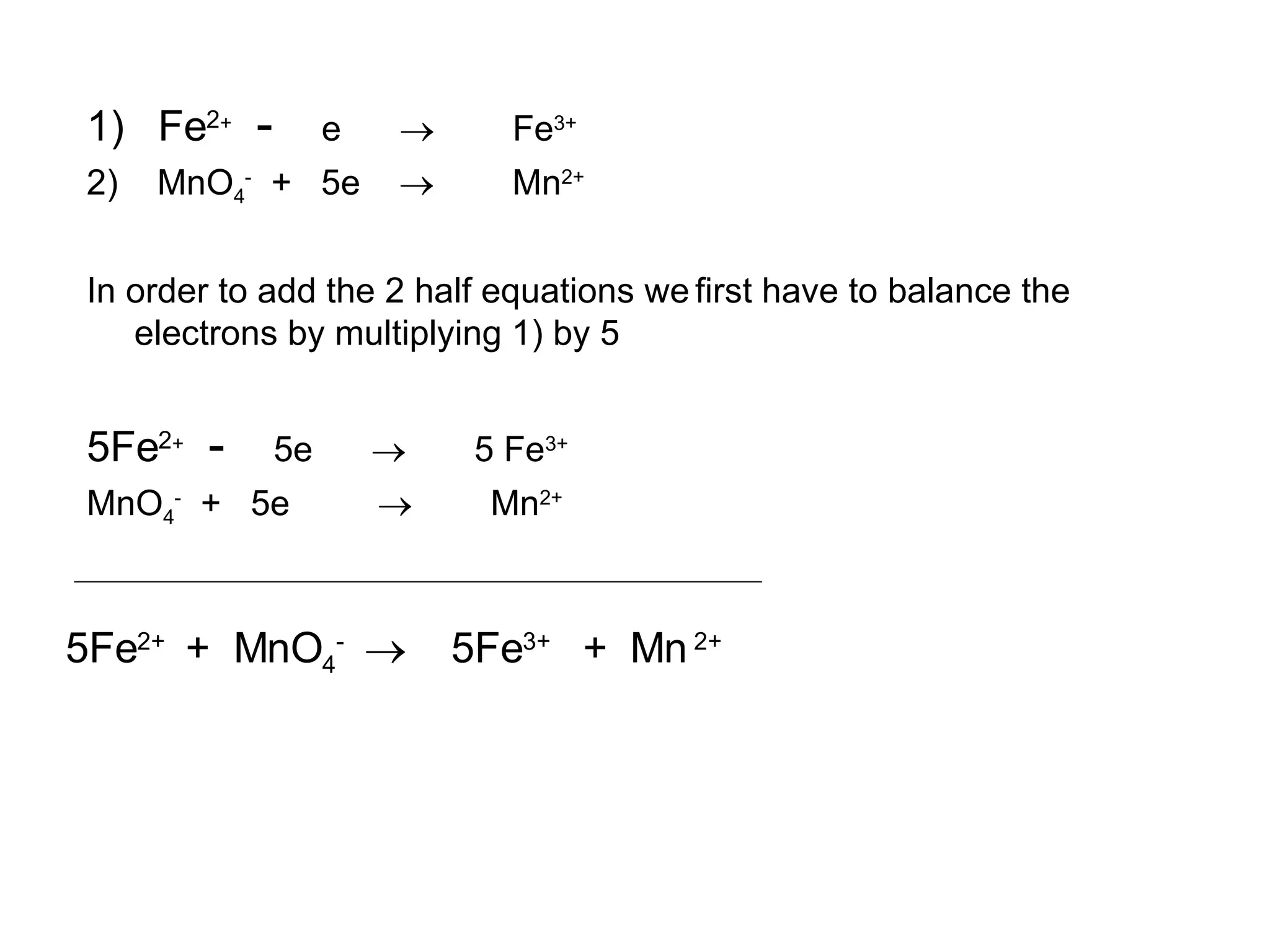
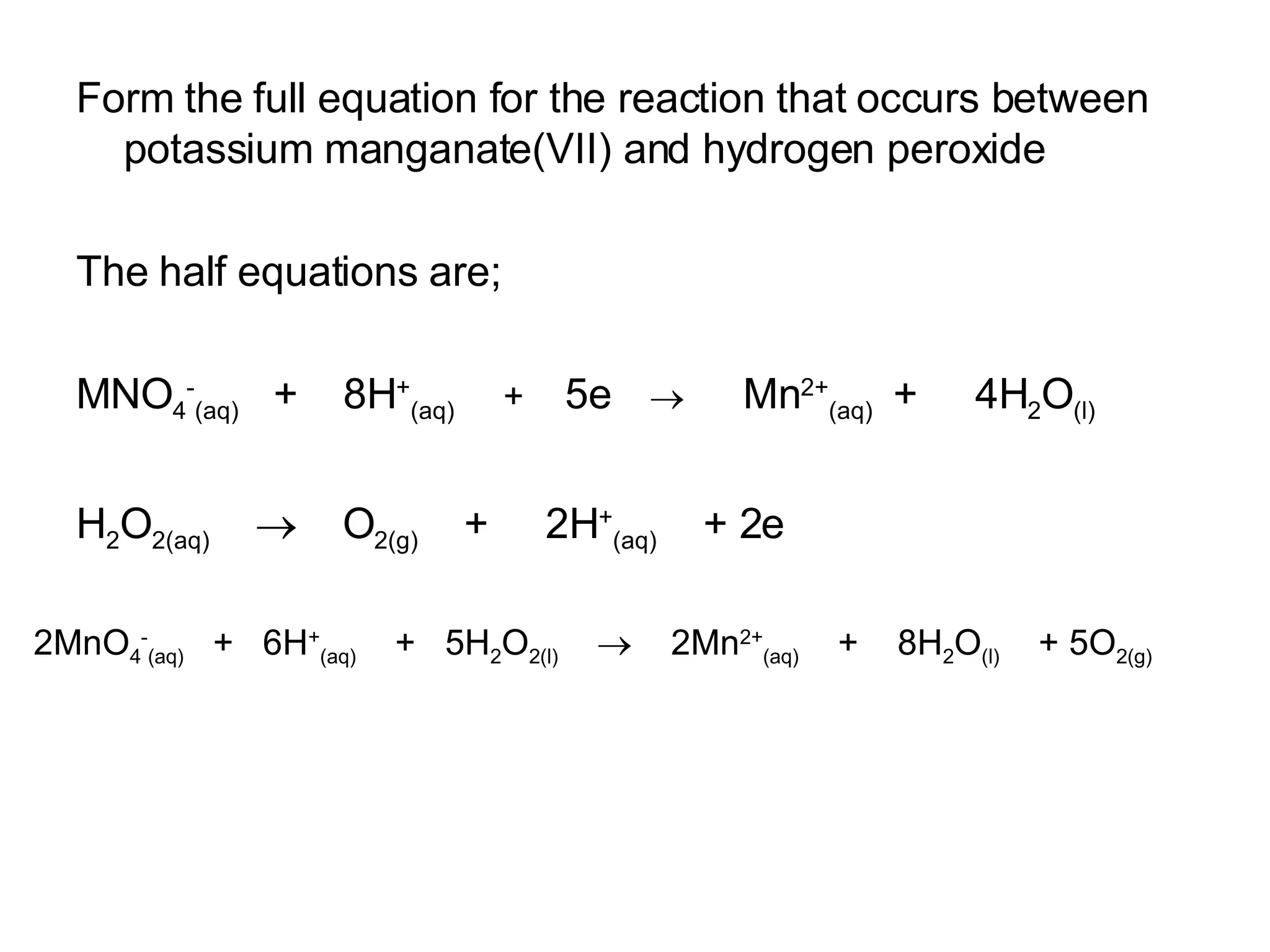
The document discusses oxidation-reduction (redox) reactions and oxidation numbers. It provides examples of half reactions and full redox reactions formed by combining half reactions. Oxidation involves an increase in oxidation state through loss of electrons, while reduction involves a decrease in oxidation state through gain of electrons. The species donating electrons is the reducing agent, while the species gaining electrons is the oxidizing agent.