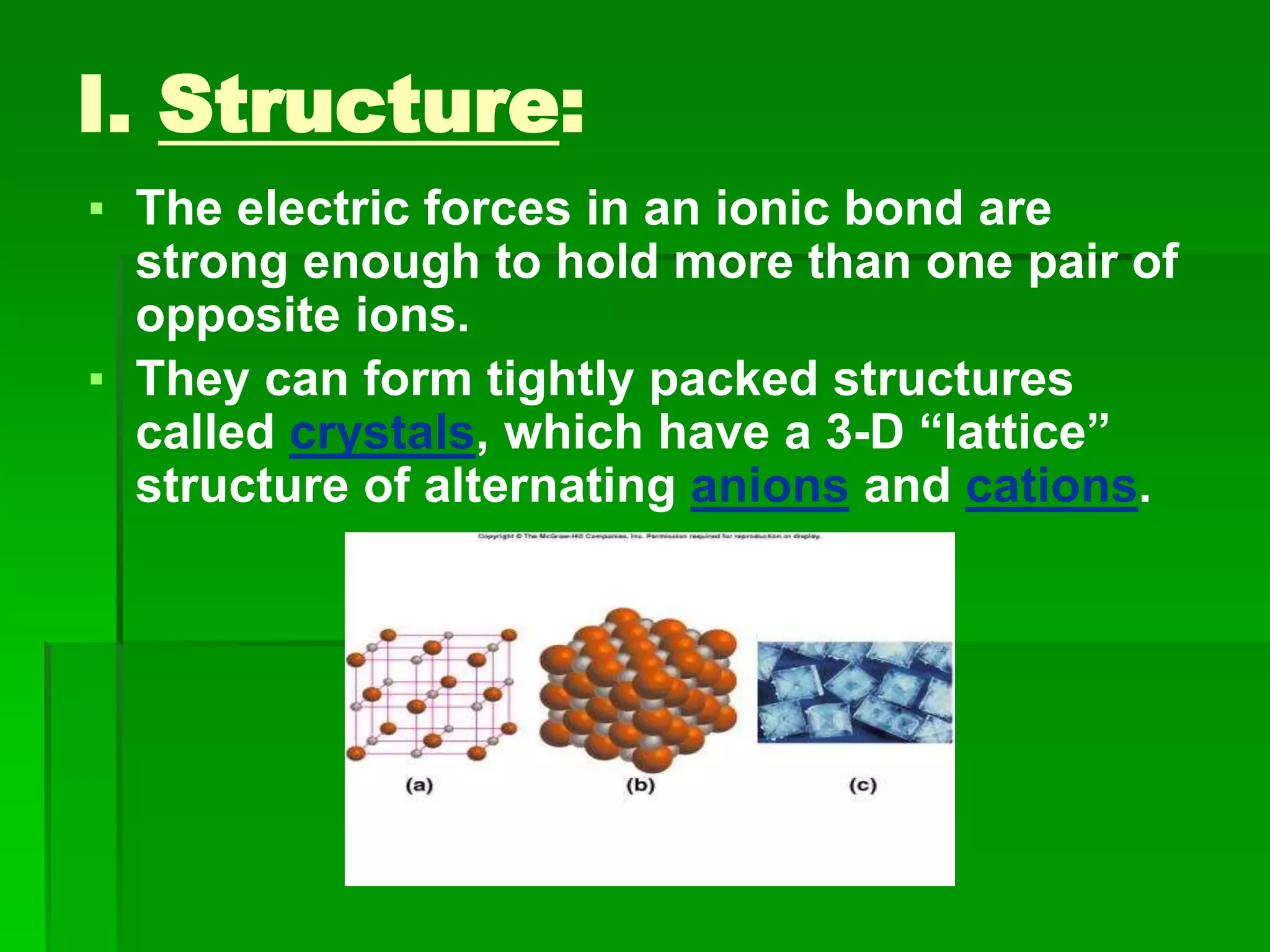
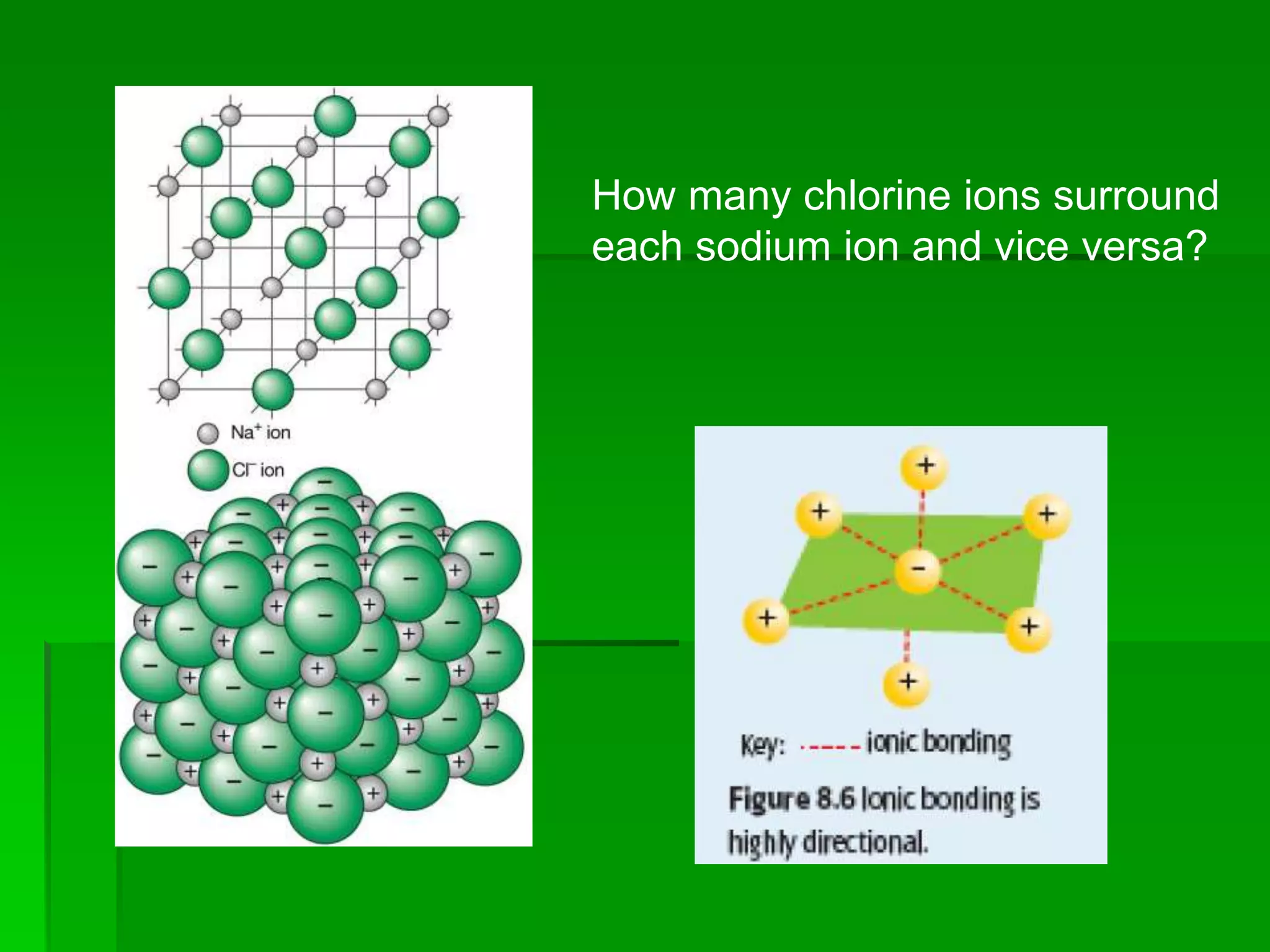
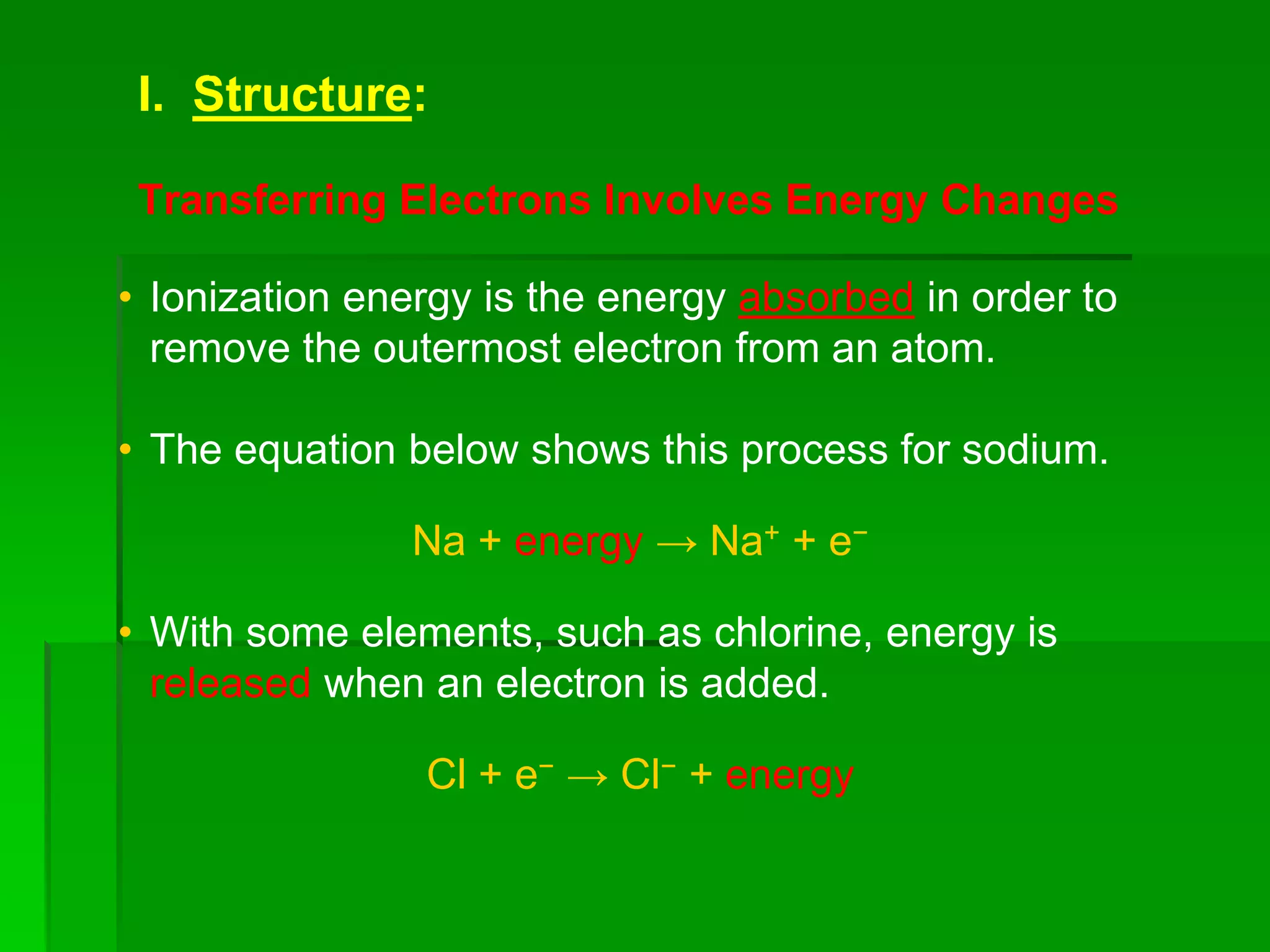
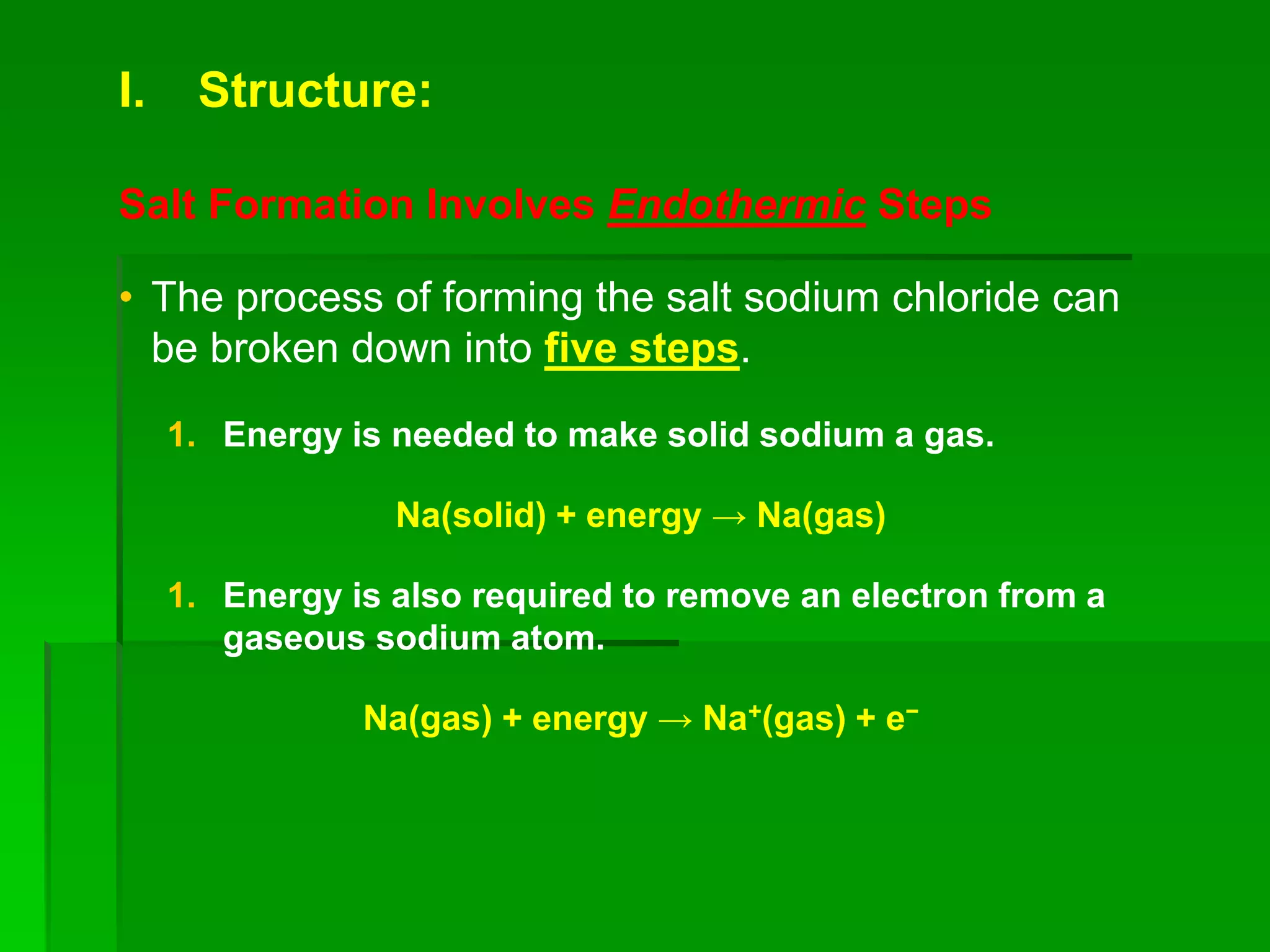
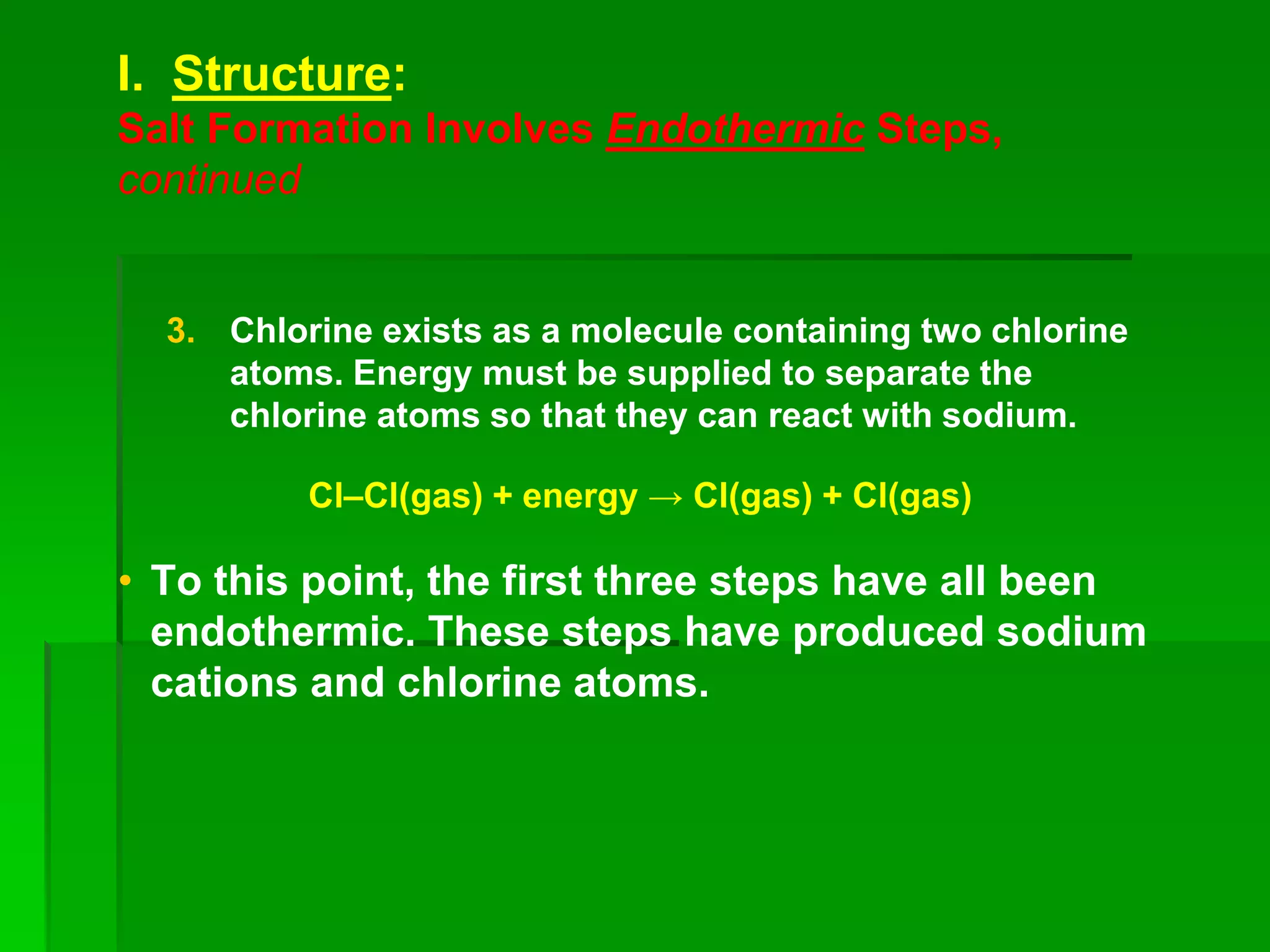
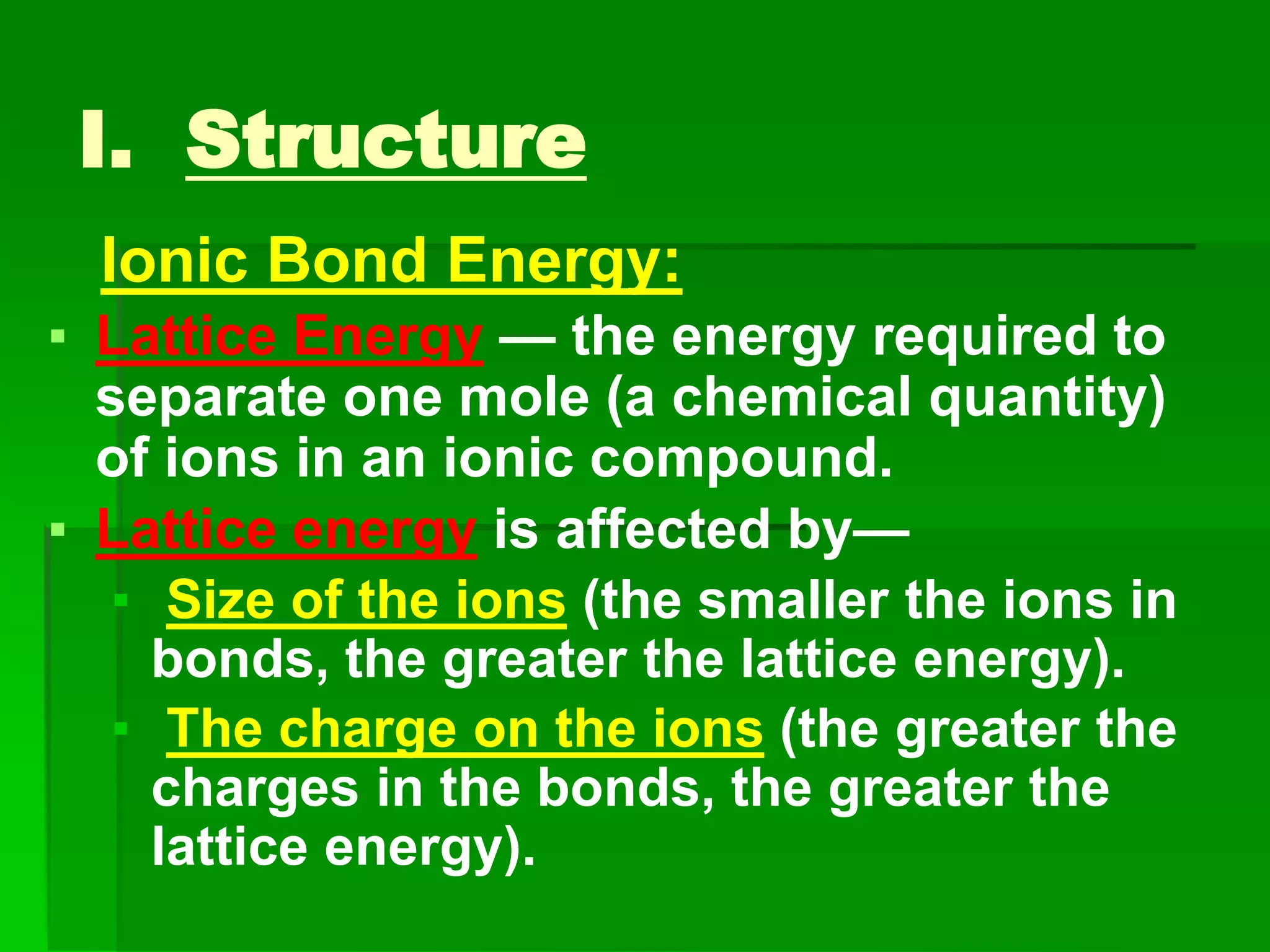
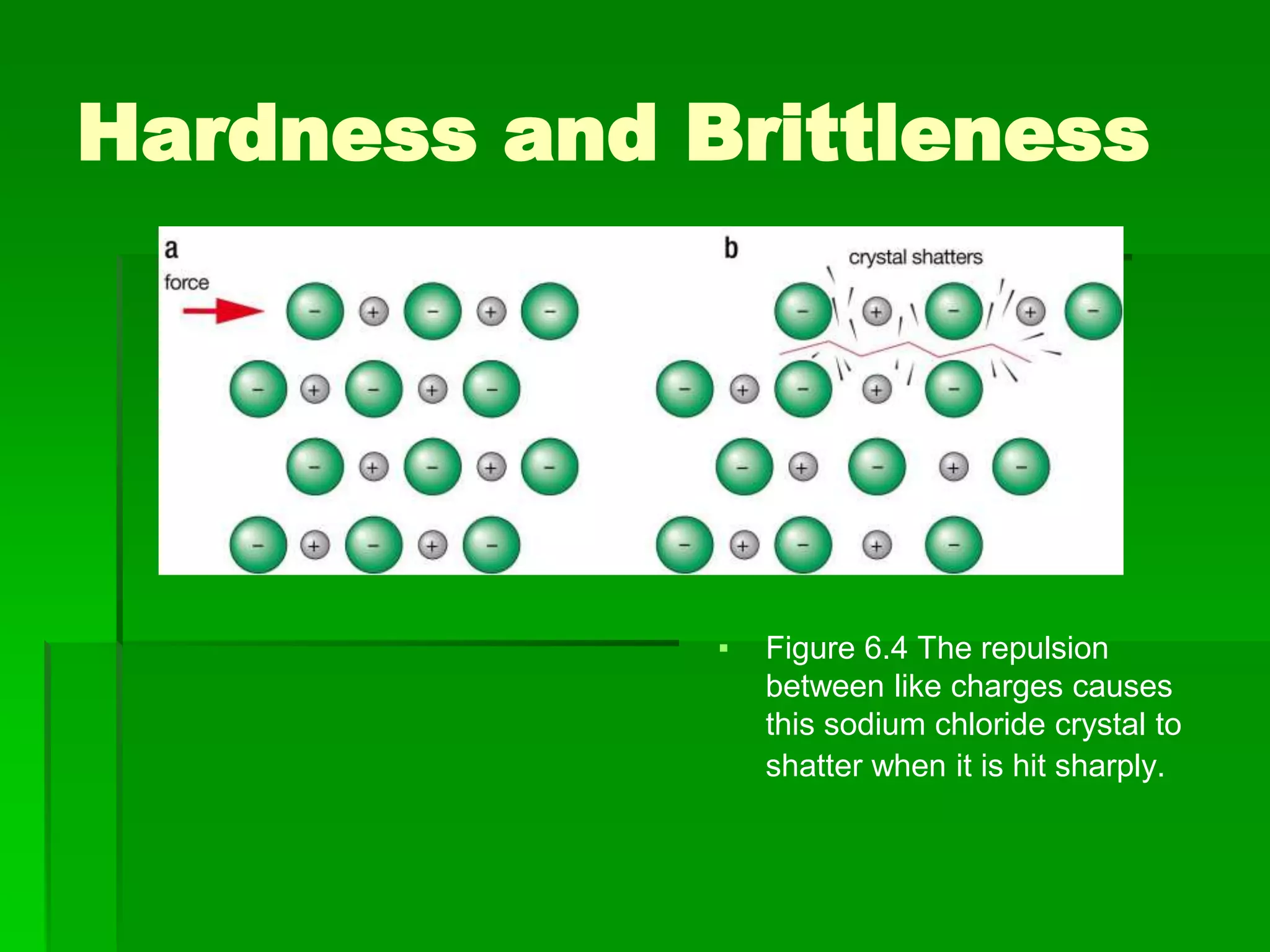
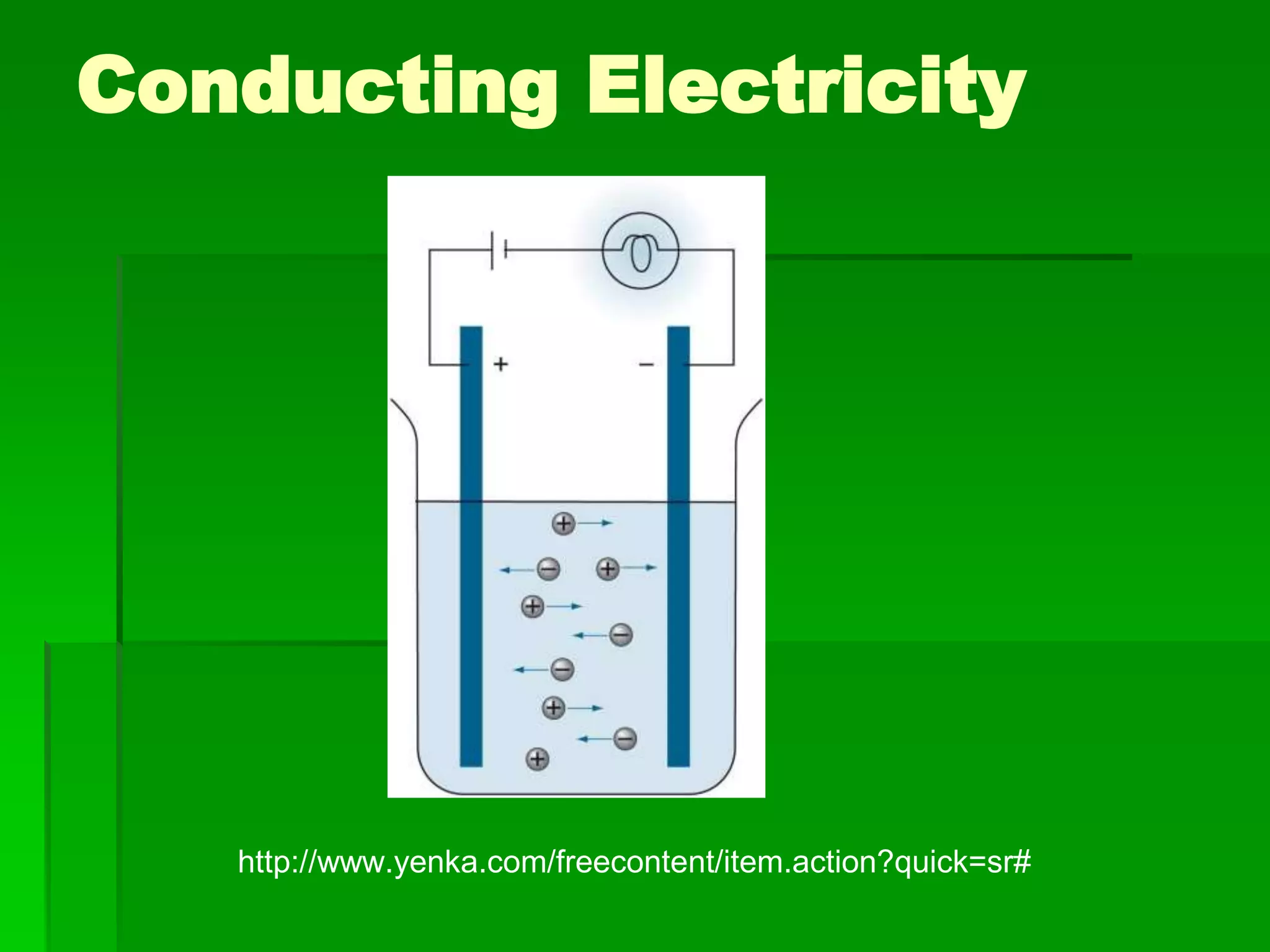
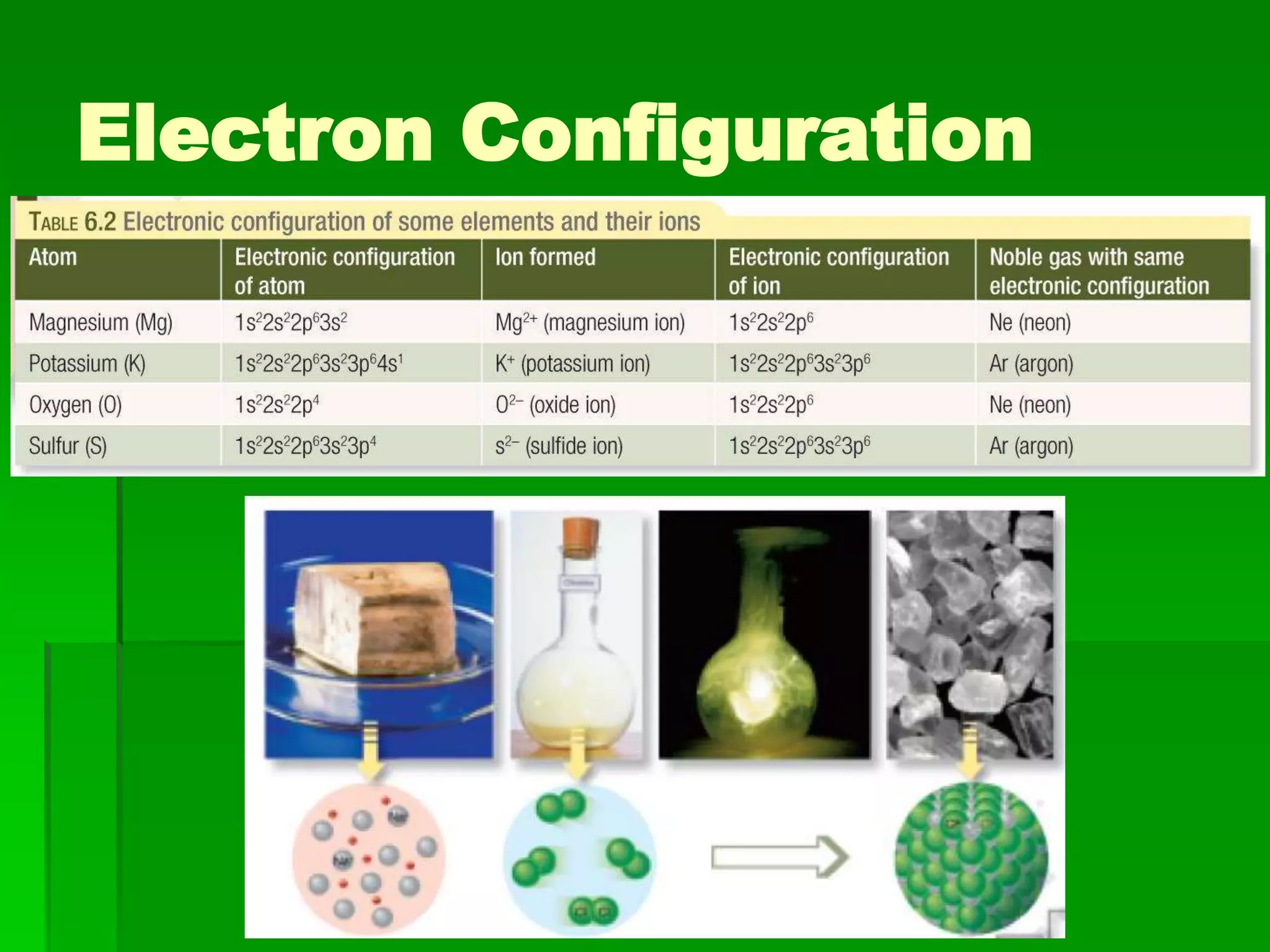
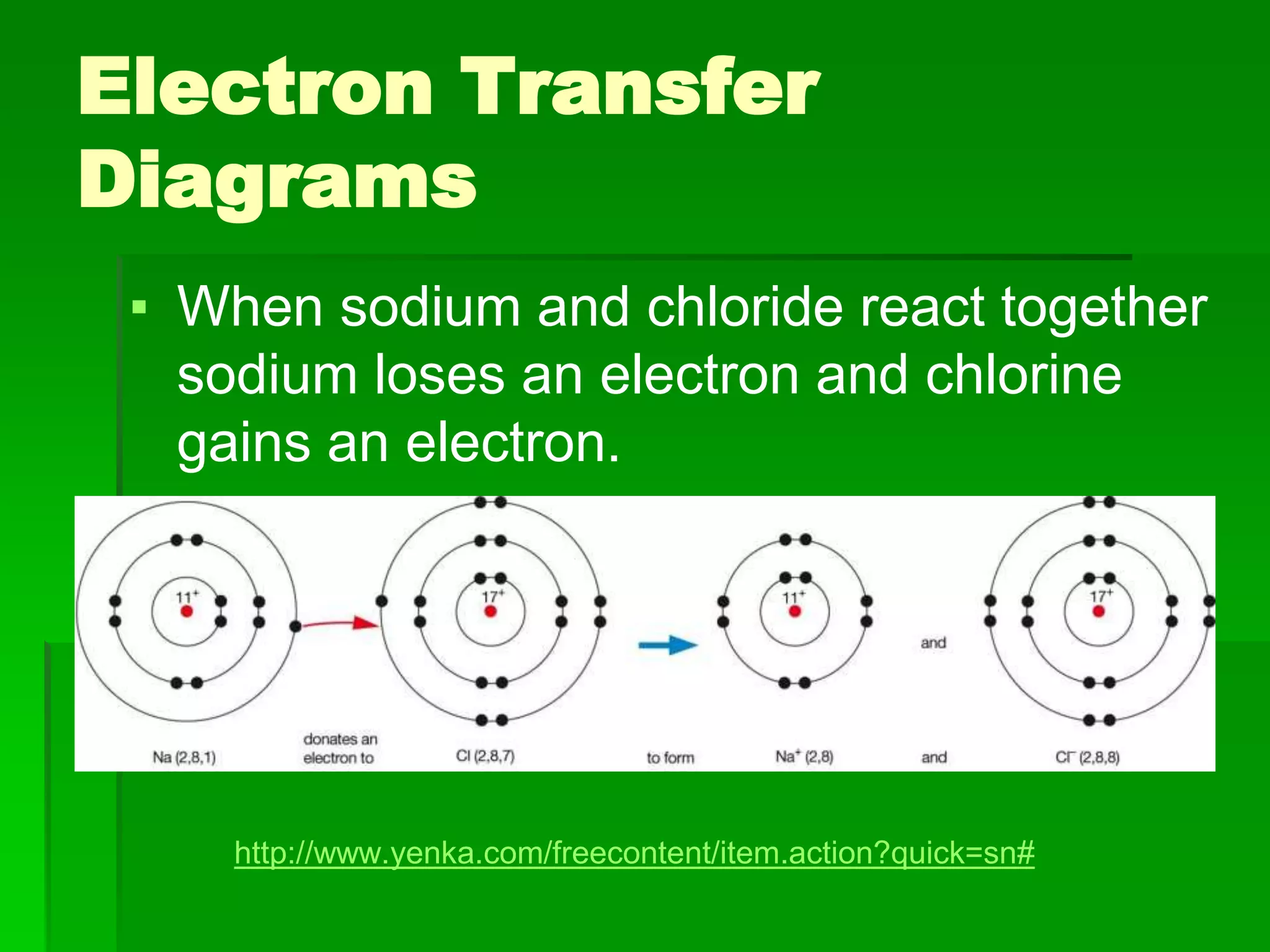
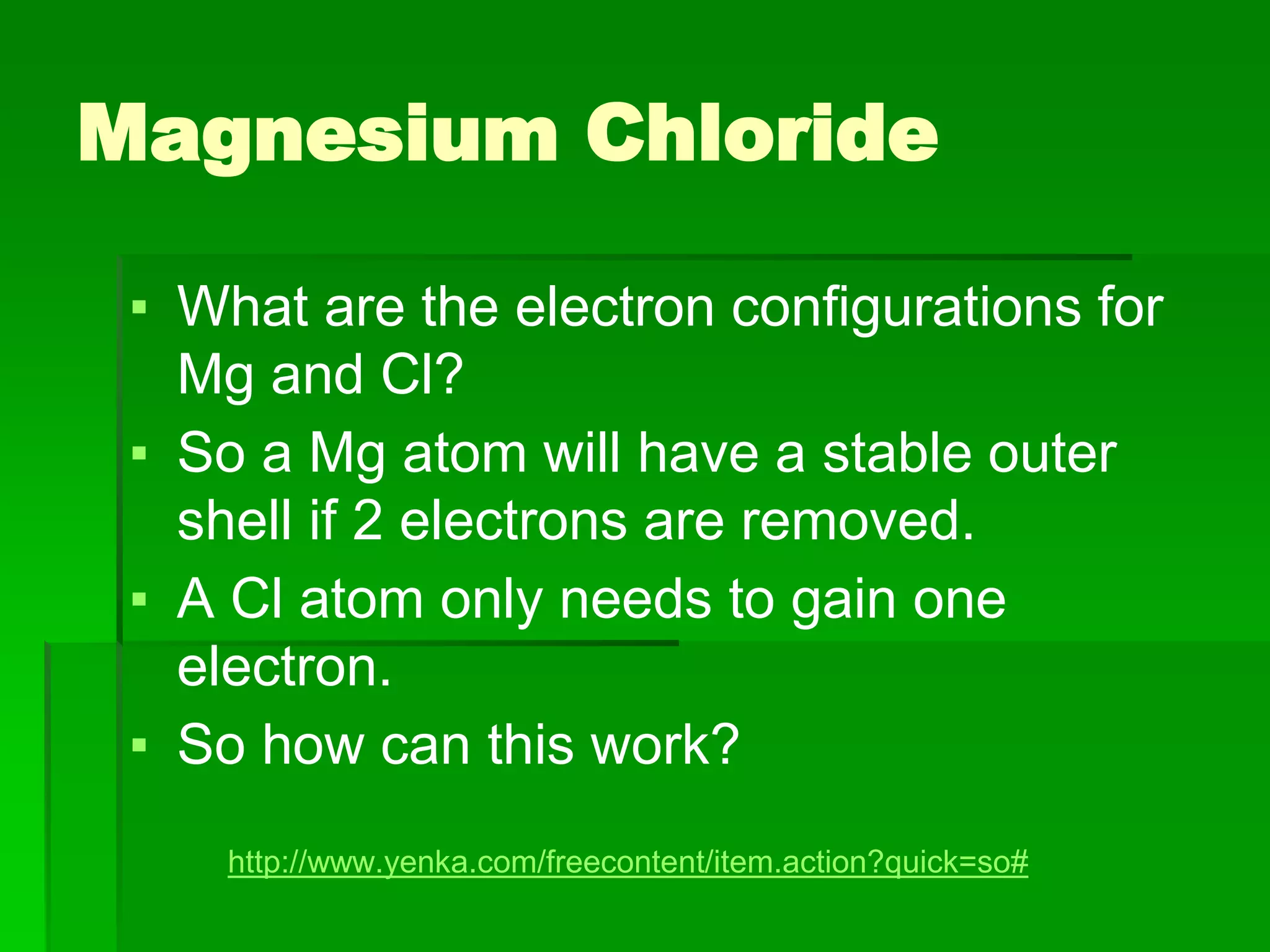
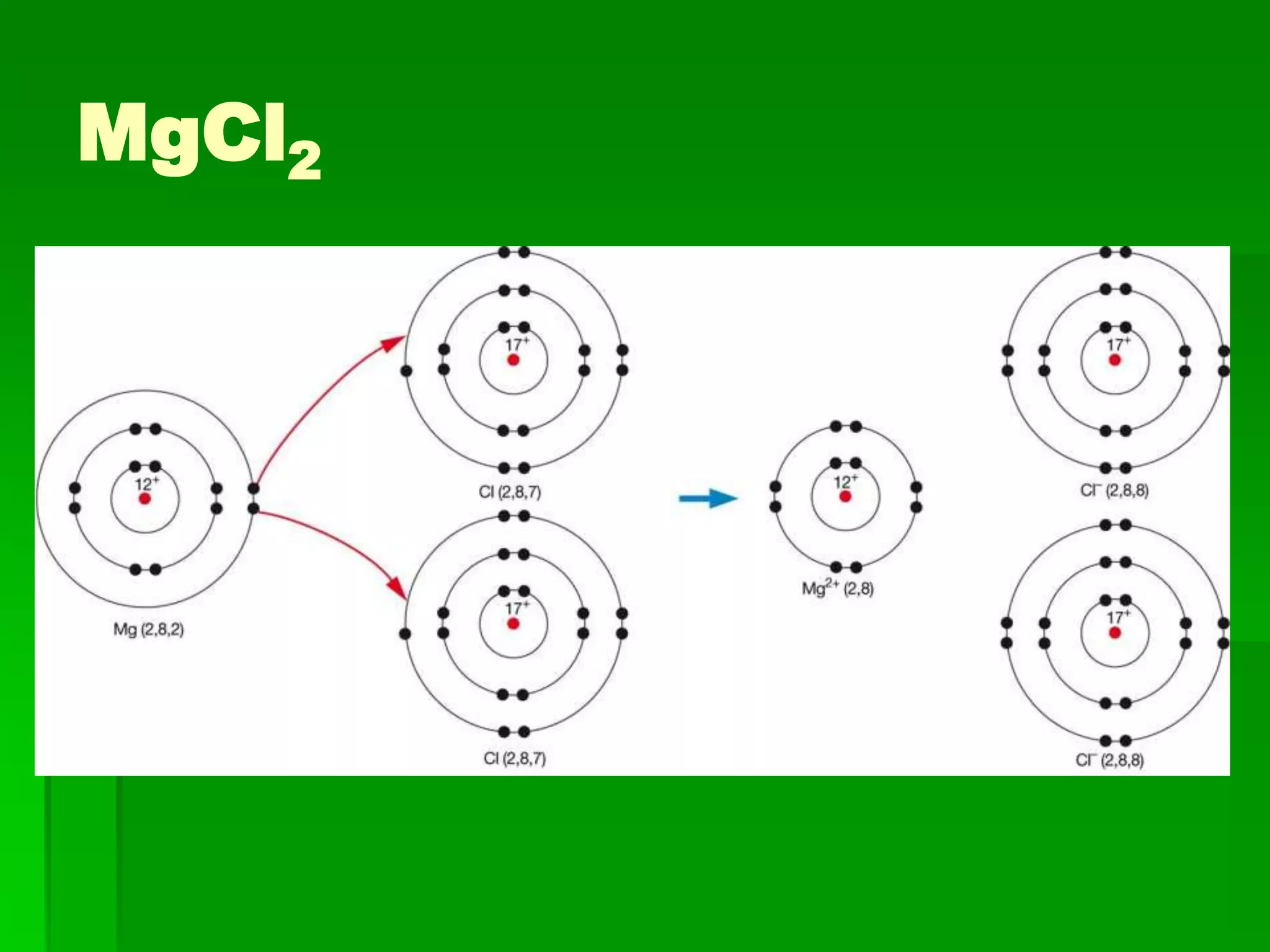
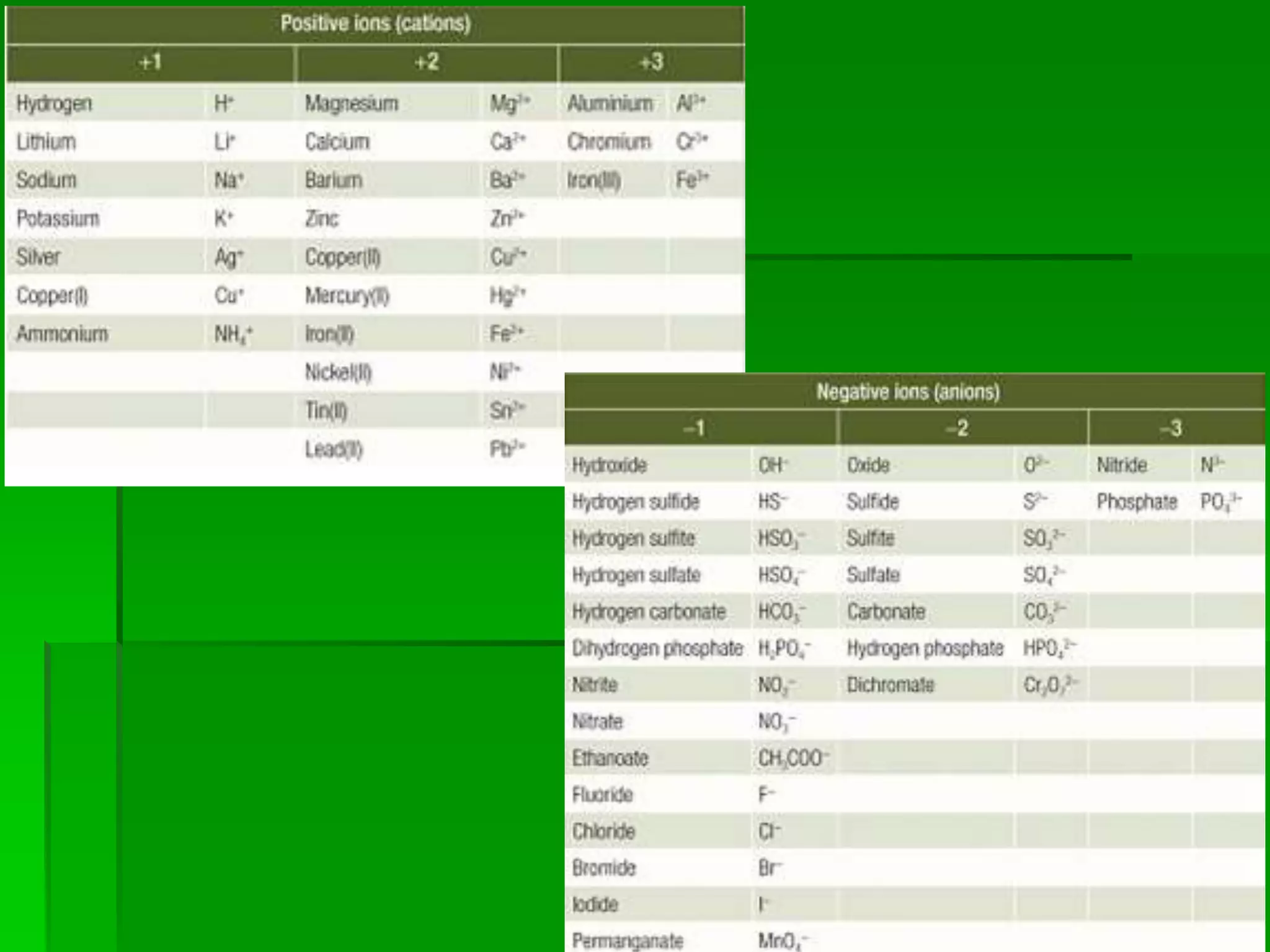
This document discusses the structure and properties of ionic compounds. It begins by explaining that ionic compounds are formed through the electrostatic attraction between oppositely charged ions. The ions form a crystalline lattice structure held together by strong ionic bonds. This lattice structure results in ionic compounds having high melting points, hardness, and brittleness. The document then discusses how ionic bonding occurs through the transfer of electrons between metals and nonmetals. It provides examples of writing chemical formulas and naming ionic compounds based on their ion charges.