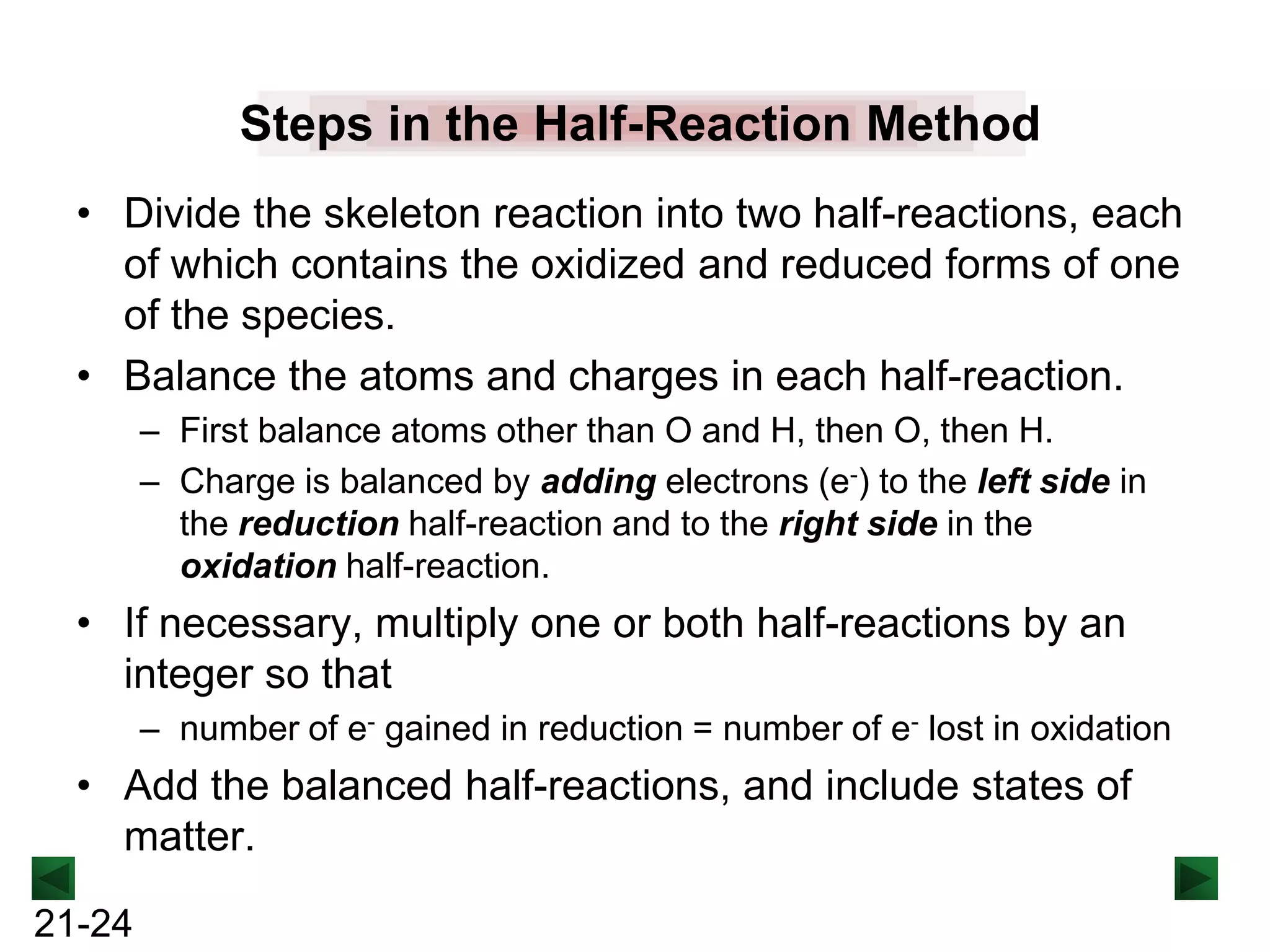
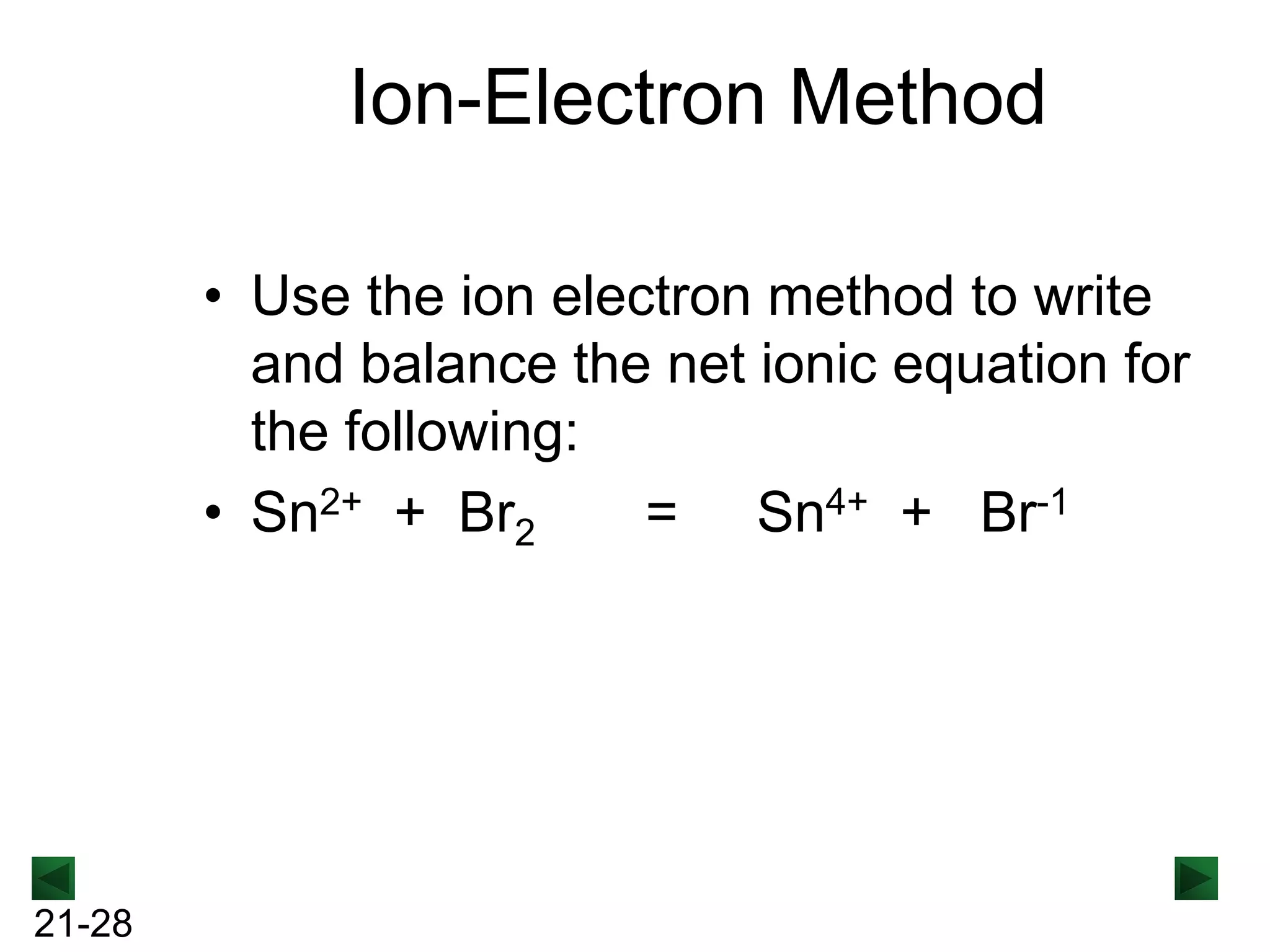
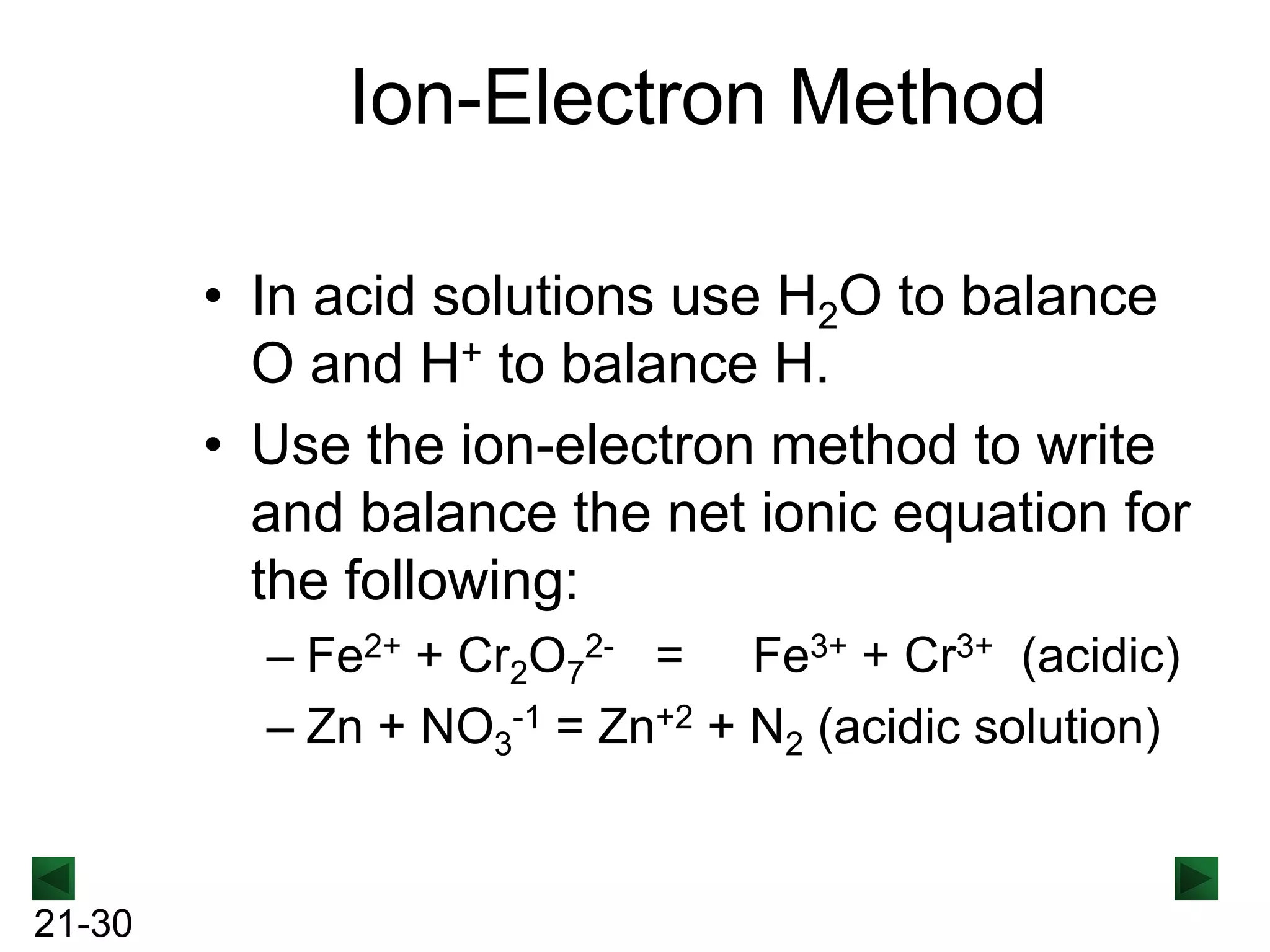
The document discusses oxidation-reduction (redox) reactions and provides information on key concepts:
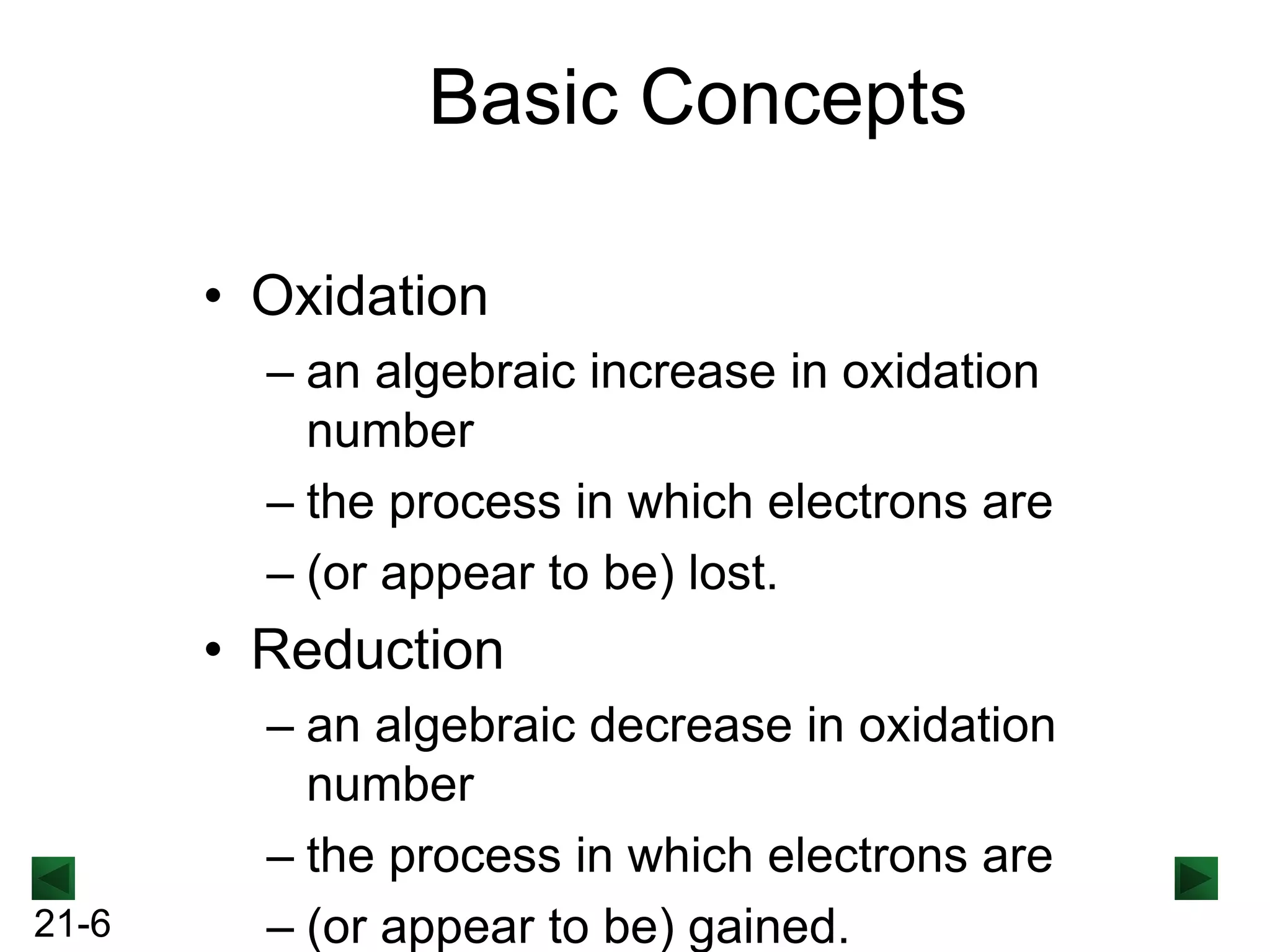
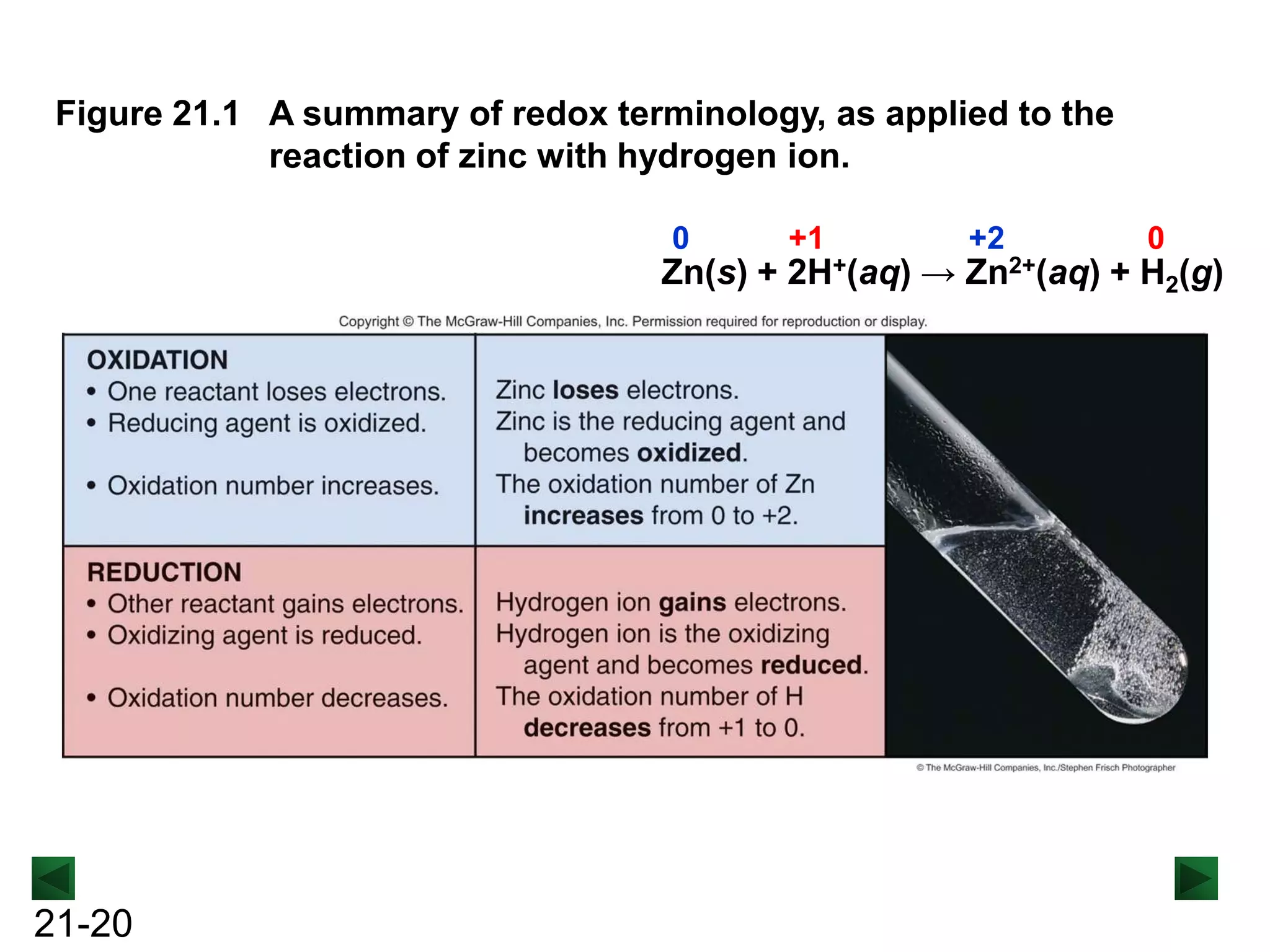
- Oxidation involves loss of electrons and increases oxidation number, reduction involves gain of electrons and decreases oxidation number.
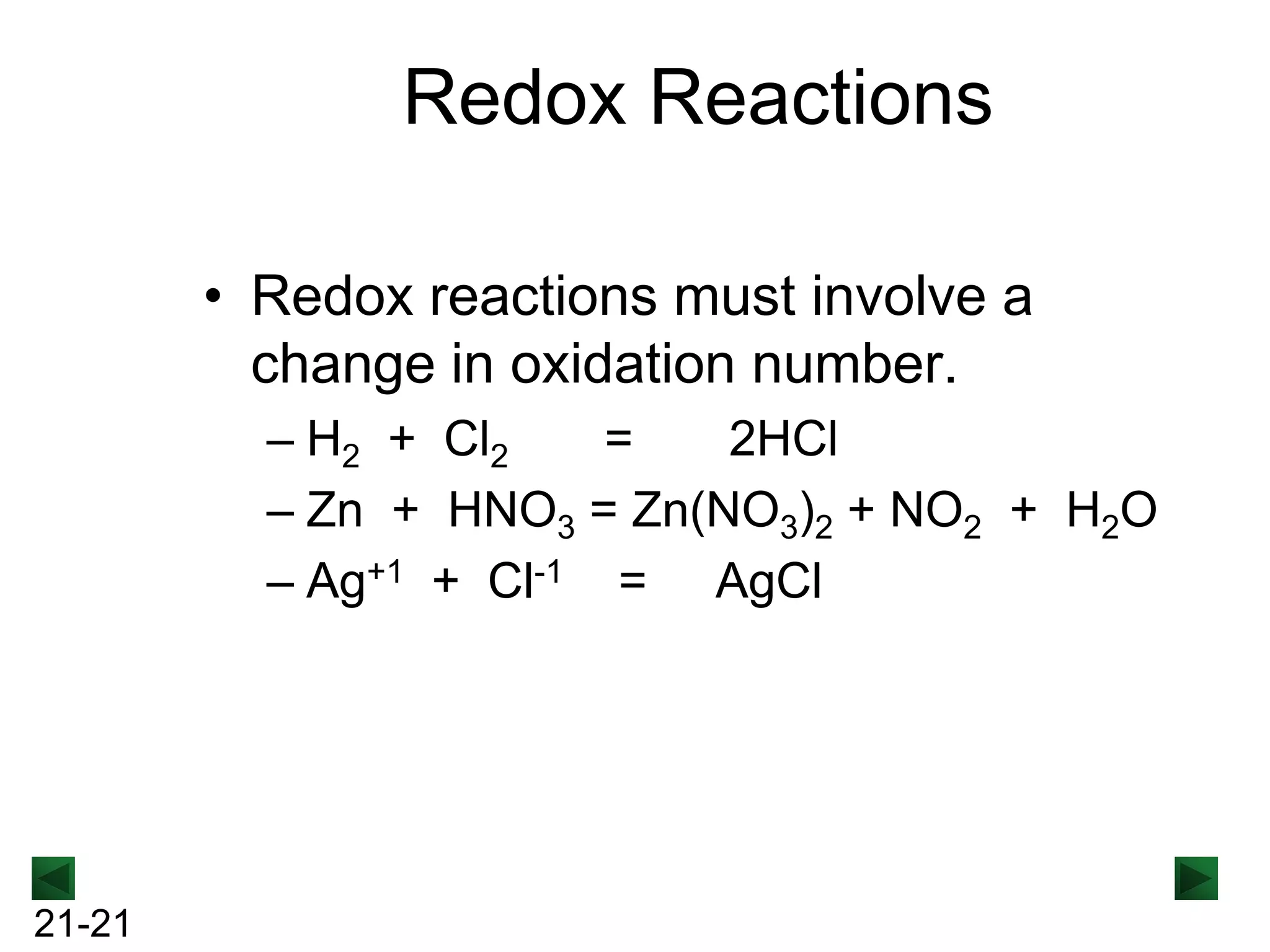
- Redox reactions involve both oxidation and reduction occurring simultaneously.
- Oxidizing agents are reduced by gaining electrons from other substances, while reducing agents are oxidized by losing electrons to other substances.


















![Sample Problem 21.1
Balancing a Redox Reaction in Basic
Solution
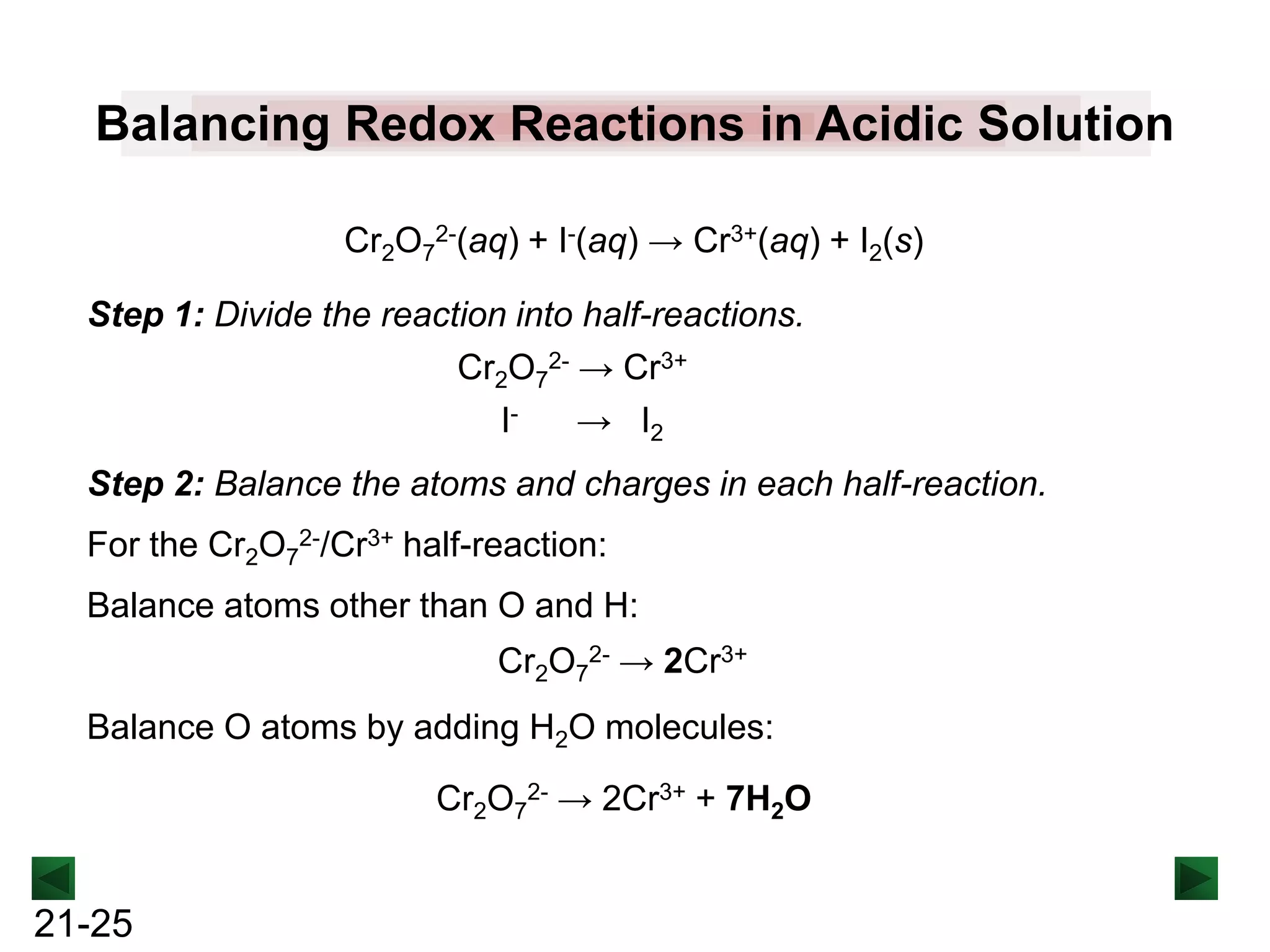
PROBLEM: Permanganate ion reacts in basic solution with oxalate
ion to form carbonate ion and solid manganese dioxide.
Balance the skeleton ionic equation for the reaction
between NaMnO4 and Na2C2O4 in basic solution:
MnO4-(aq) + C2O42-(aq) → MnO2(s) + CO32-(aq) [basic solution]
PLAN: We follow the numbered steps as described in the text, and
proceed through step 4 as if this reaction occurs in acidic
solution. Then we add the appropriate number of OH- ions
and cancel excess H2O molecules.
SOLUTION:
Step 1: Divide the reaction into half-reactions.
MnO4- → MnO2
C2O42- → CO32-
21-34](https://image.slidesharecdn.com/new-20chm-20152-20unit-207-20power-20points-su13-140227172225-phpapp02/75/New-chm-152-unit-7-power-points-su13-34-2048.jpg)
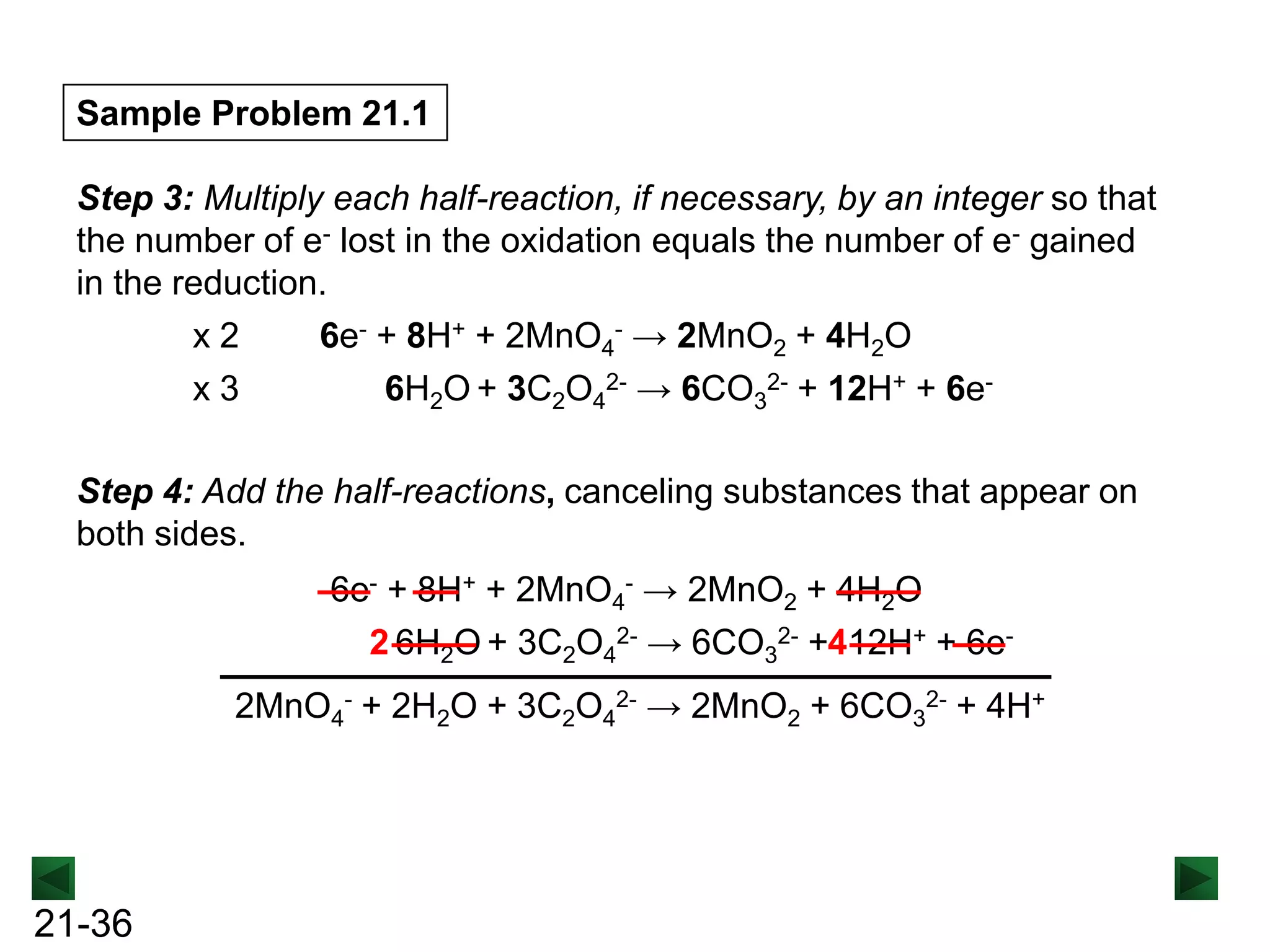
![Sample Problem 21.1
Step 2: Balance the atoms and charges in each half-reaction.
Balance atoms other than O and H:
MnO4- → MnO2
C2O42- → 2CO32-
Balance O atoms by adding H2O molecules:
MnO4- → MnO2 + 2H2O
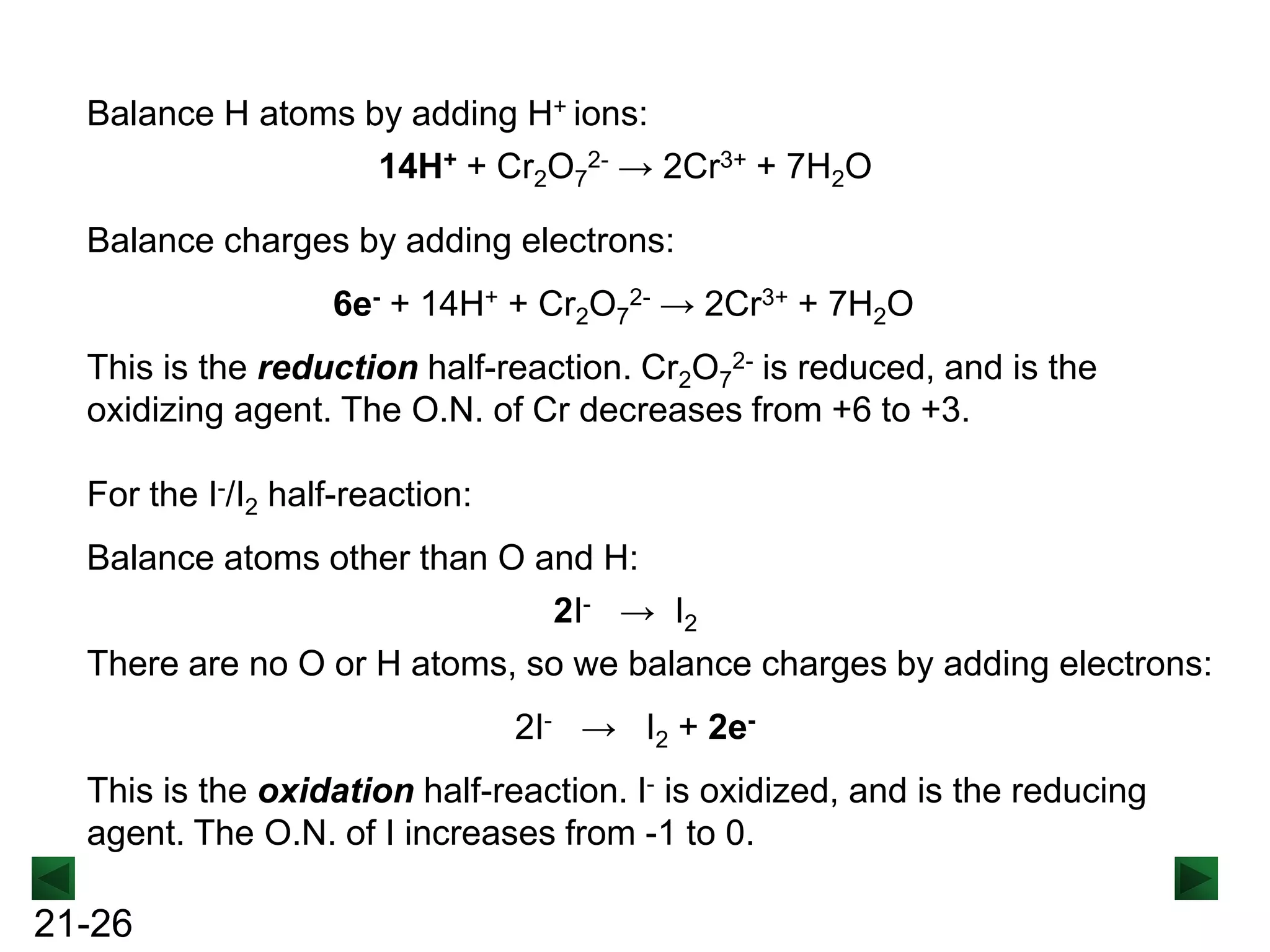
2H2O + C2O42- → 2CO32Balance H atoms by adding H+ ions:
4H+ + MnO4- → MnO2 + 2H2O
2H2O + C2O42- → 2CO32- + 4H+
Balance charges by adding electrons:
3e- + 4H+ + MnO4- → MnO2 + 2H2O 2H2O + C2O42- → 2CO32- + 4H+ + 2e[reduction]
[oxidation]
21-35](https://image.slidesharecdn.com/new-20chm-20152-20unit-207-20power-20points-su13-140227172225-phpapp02/75/New-chm-152-unit-7-power-points-su13-35-2048.jpg)
![Sample Problem 21.1
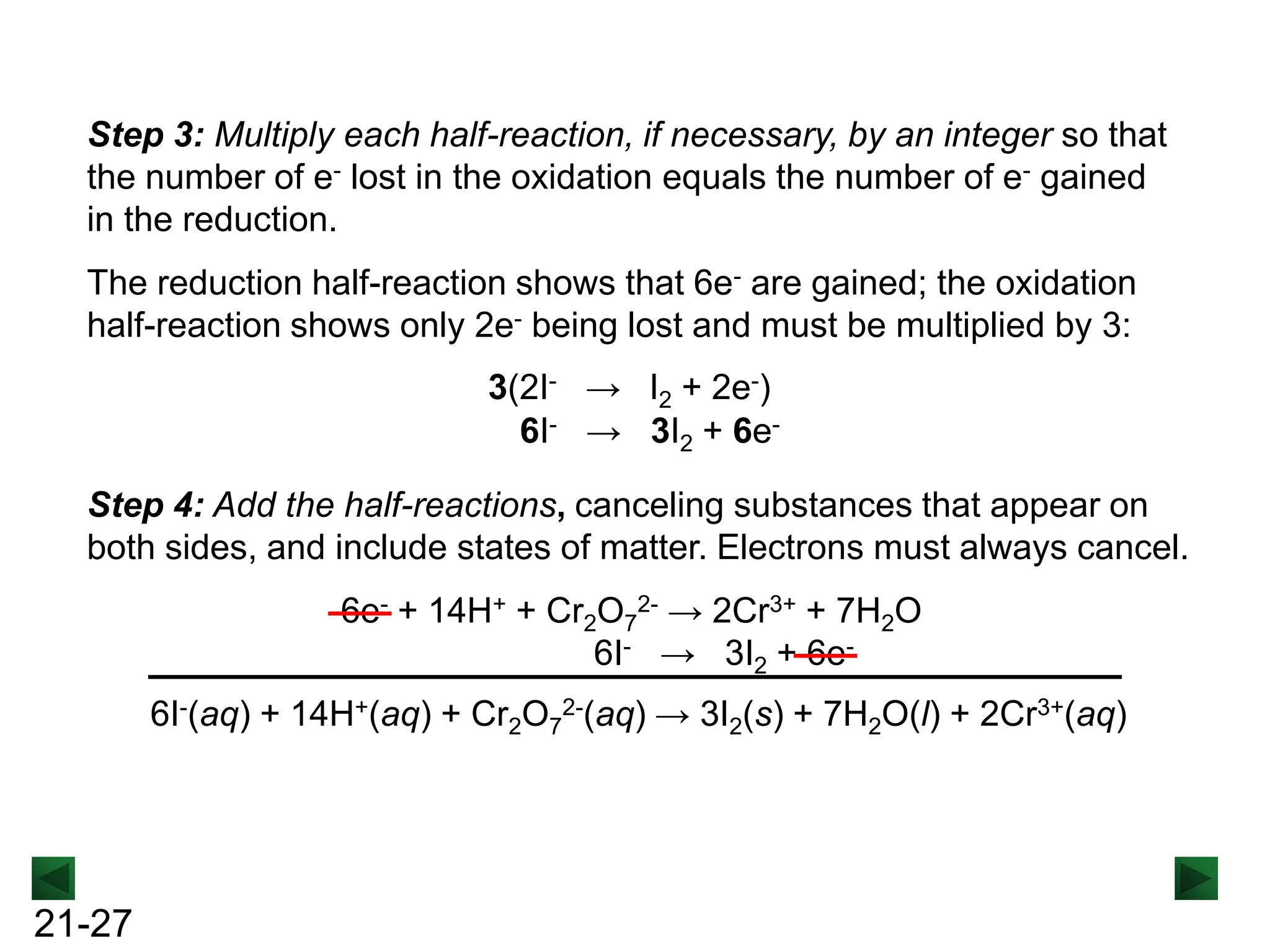
Basic. Add OH- to both sides of the equation to neutralize H+, and
cancel H2O.
2MnO4- + 2H2O + 3C2O42- + 4OH- → 2MnO2 + 6CO32- + [4H+ + 4OH-]
2MnO4- + 2H2O + 3C2O42- + 4OH- → 2MnO2 + 6CO32- + 2 4H2O
Including states of matter gives the final balanced equation:
2MnO4-(aq) + 3C2O42-(aq) + 4OH-(aq) → 2MnO2(s) + 6CO32-(aq) + 2H2O(l)
21-37](https://image.slidesharecdn.com/new-20chm-20152-20unit-207-20power-20points-su13-140227172225-phpapp02/75/New-chm-152-unit-7-power-points-su13-37-2048.jpg)


