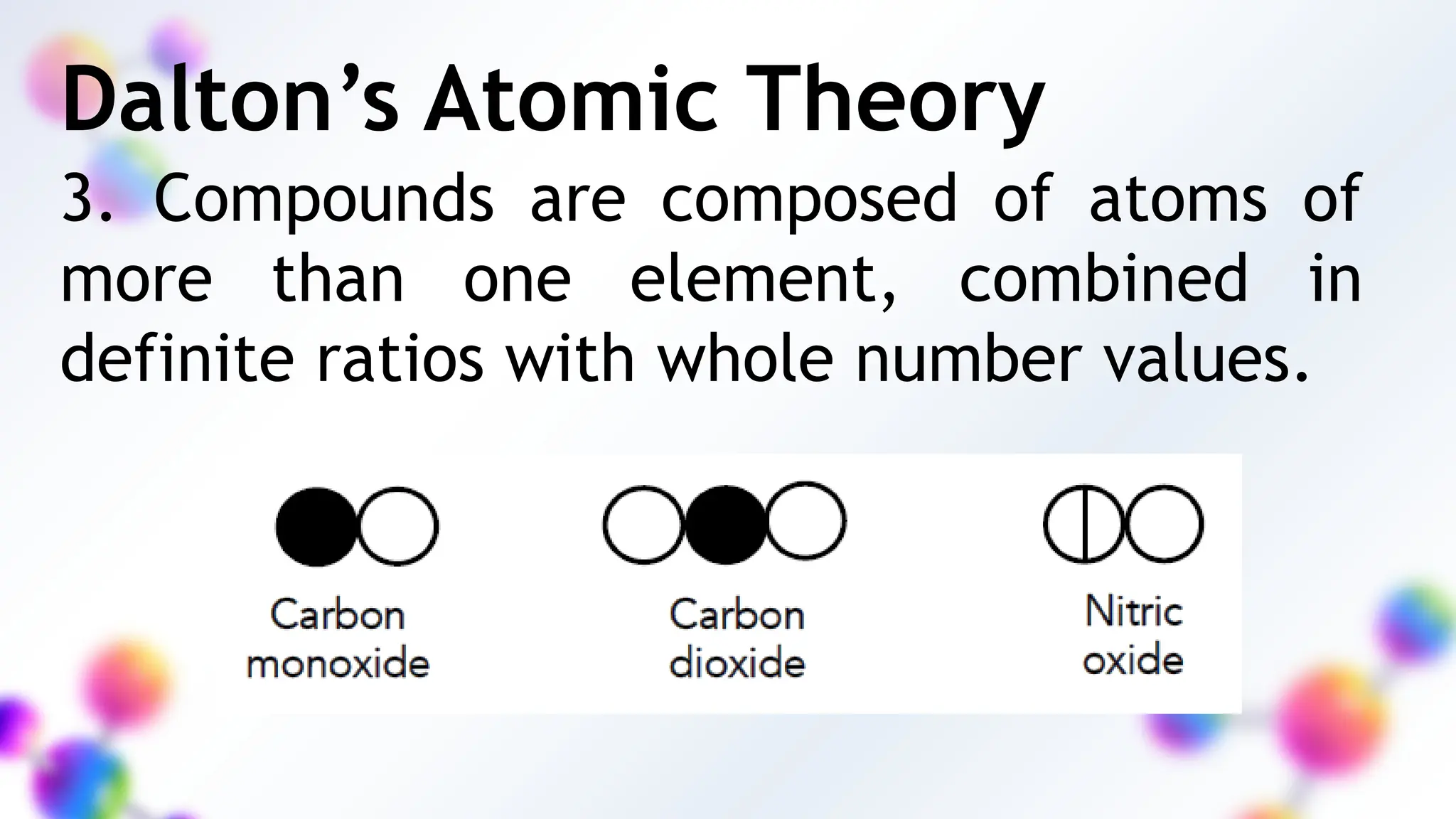
The document explores the historical development of atomic theory, starting from ancient Greek philosophers to modern discoveries, highlighting key scientists like Dalton, Thomson, and Rutherford. It discusses various laws of chemistry such as the conservation of mass, definite proportions, and multiple proportions, while defining the structure of atoms and the concept of isotopes. Additionally, the document explains the formation and naming conventions of ionic and molecular compounds.