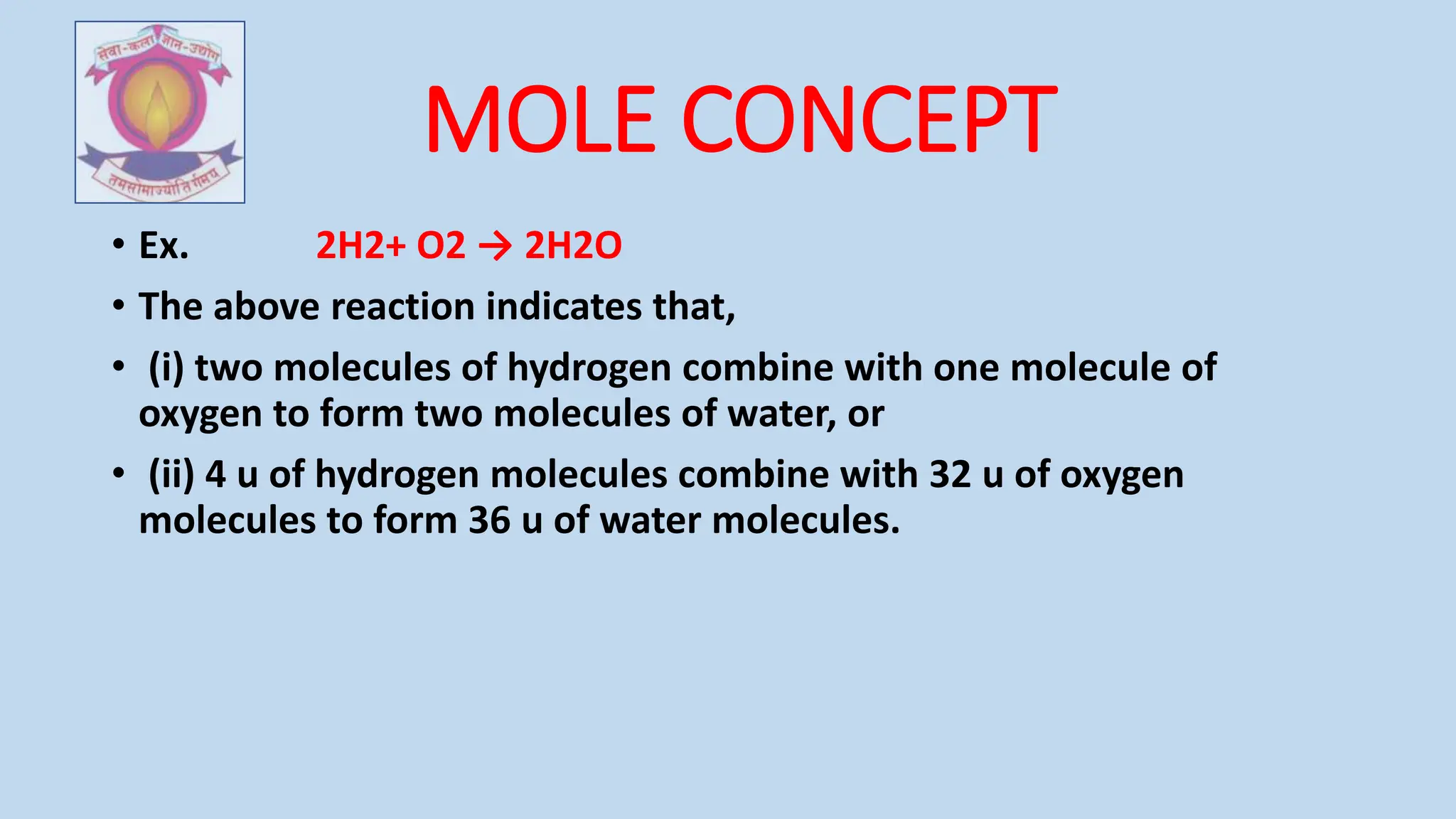
The document discusses the foundational concepts of atoms and molecules, tracing their philosophical origins from ancient Indian and Greek philosophers to modern scientific understanding. It covers key laws of chemical combination, Dalton's atomic theory, and the significance of atomic mass and chemical formulae, emphasizing the relationships between elements and compounds. Additionally, it explains the mole concept, valency, molecular mass, and the practical applications of these principles in chemistry.