1. Atoms are the smallest particles that make up elements, consisting of a nucleus surrounded by electrons. John Dalton established the first atomic theory in 1808.
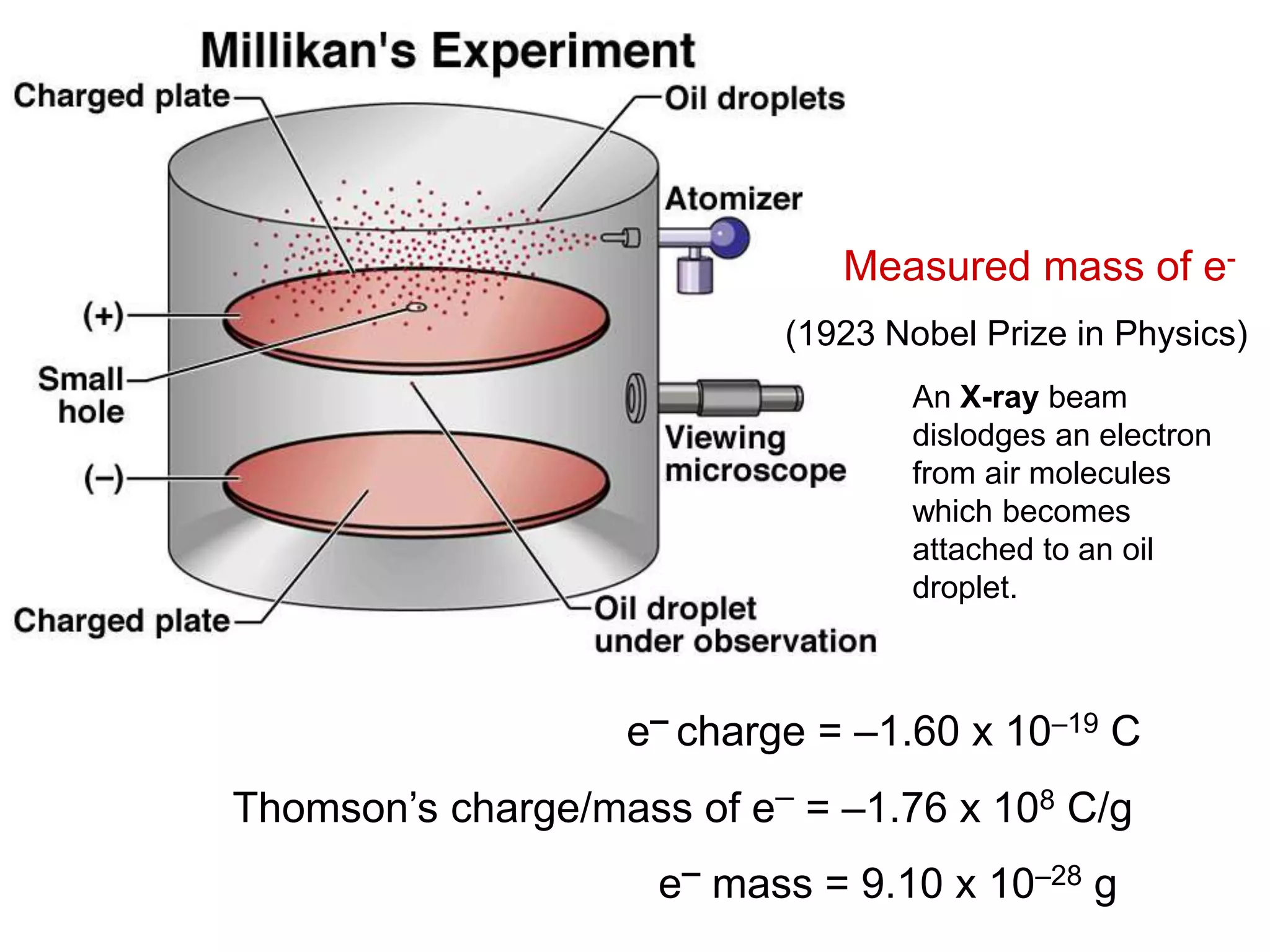
2. In the early 20th century, experiments by Thomson, Rutherford, and Chadwick led to discoveries about the internal structure of atoms, including the identification of protons, electrons, and neutrons within the nucleus.
3. Isotopes are atoms of the same element that differ in their number of neutrons. Molecules, ions, and compounds are formed through combinations and interactions between atoms.