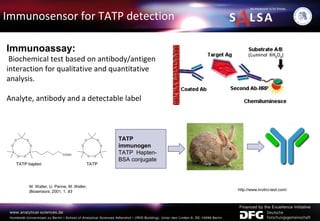
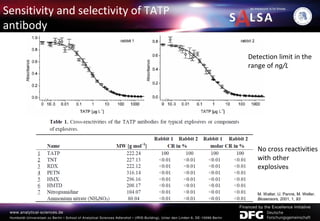
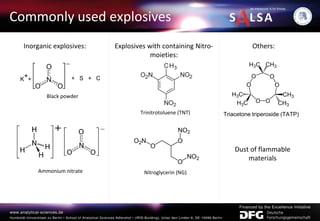
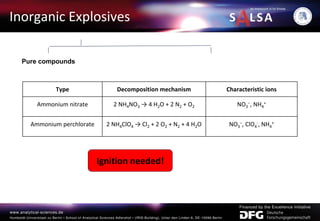
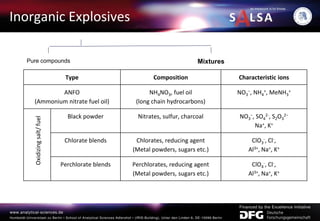
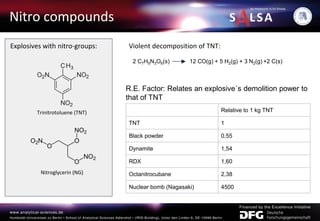
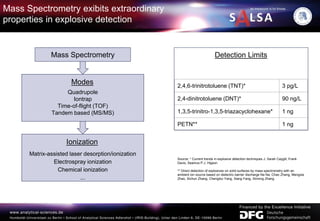
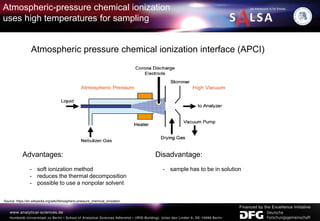
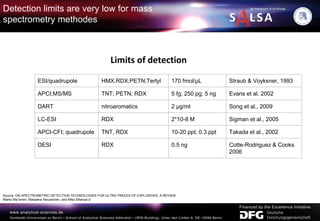
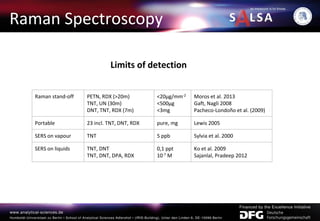
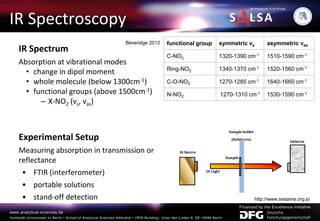
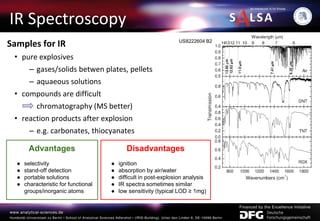
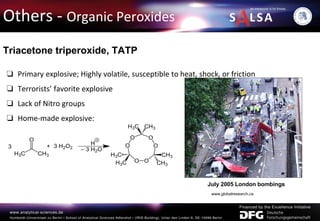
The document presents an extensive overview of forensic analysis techniques for detecting and identifying explosives, highlighting both inorganic and organic explosives, their preparation methods, and analytical techniques used in forensic contexts. It details various explosives such as ammonium nitrate, TNT, and TATP, and discusses analytical methods including mass spectrometry, spectroscopy, colorimetric reactions, and immunoassays. The document emphasizes the importance of these techniques in areas like airport security, demining, and post-explosion analysis.





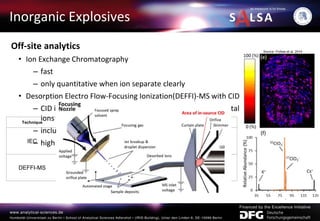
![Direct Analysis in Real Time is very useful for
examining surfaces
Direct Analysis in Real Time (DART)
Mechanism in Detail: Penning Ionization
M*+ S S+• + M + e-
He(23S) + H2O H2O+•+ He(11S) + electron
H2O+•+H2O H3O++ OH•
H3O++ n H2O [(H2O)nH]+
[(H2O)nH]++ S SH++nH2O
Source: Direct Analysis in Real Time (DARTtm) Mass Spectrometry Robert B. Cody, James A. Laramée, J. Michael Nilles, H. Dupont Durst
Sample](https://image.slidesharecdn.com/031115-presentationexplosives-240814070614-68339cec/85/031115-Presentation_chemistryExplosives-pdf-13-320.jpg)



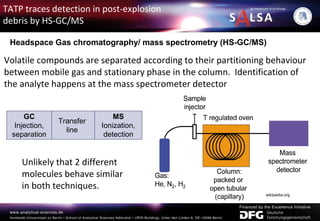
![Headspace sampling:
analysis of the gas phase in the headspace above the sample.
Post-explosion debris (soil, glass and metals) collected from the
area of explosion in a glass container and heated. Then, a sample
from the headspace is injected to GC/MS.
m/z [M-1]
Detection limit of
1 nanogram
TATP traces detection in post-explosion
debris by HS-GC/MS
Stambouli A. et al., Forensic Sci
Int., 2004, 146S, S191
http://www.labhut.com/](https://image.slidesharecdn.com/031115-presentationexplosives-240814070614-68339cec/85/031115-Presentation_chemistryExplosives-pdf-24-320.jpg)