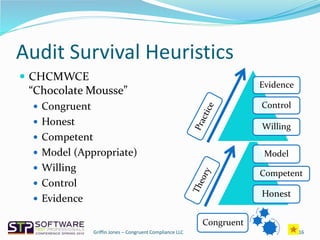
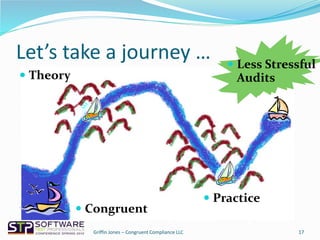
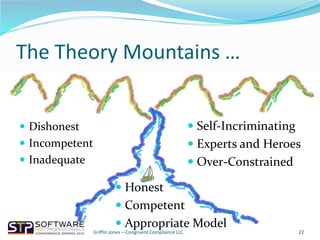
The document outlines strategies for navigating FDA audits in relation to exploratory testing (ET), emphasizing the need for clear communication, adequate documentation, and sufficient evidence to satisfy regulatory auditors. It highlights the importance of adjusting to the auditors' perspectives and requirements while maintaining a proactive approach to compliance through the congruence triad of honesty, competence, and appropriate modeling. The key takeaway is that challenges arise not from the regulations themselves, but from how organizations deal with them.