amide.pdf
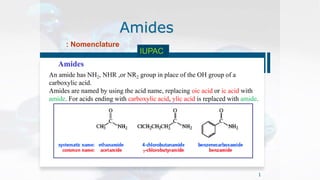
- 1. Amides Amides 1 : Nomenclature IUPAC An amide has NH2, NHR ,or NR2 group in place of the OH group of a carboxylic acid. Amides are named by using the acid name, replacing oic acid or ic acid with amide. For acids ending with carboxylic acid, ylic acid is replaced with amide.
- 2. Amides Amides 2 : Nomenclature IUPAC In case of 2ry and 3ry amides, the name of substituent is preceded by N.
- 3. Nitriles Nitriles 3 : Nomenclature IUPAC They are considered carboxylic acid derivatives because they react with water to form carboxylic acids. Nitriles are named by adding the suffix -nitrile to the alkane name. The nitrile carbon is assigned position 1 ***Ethanenitrile is usually called acetonitrile
- 4. Nitriles Nitriles 4 : Nomenclature IUPAC Nitriles can also be named as alkyl cyanides-starting the name by the alkyl group that is attached to the CN group.
- 5. Amides 5 : Preparation 1) From Acyl Chlorides Ammonia, 1ry or 2ry amines react with acid chlorides to form amides. An excess of amine is added to neutralize the HCl formed in the reaction. Carboxylic acids can be converted to amides via the corresponding acid chloride
- 6. Amides 6 : Preparation 2) From Carboxylic Anhydrides Anhydrides react with 2 equivalents of amine to produce an amide & ammonium carboxylate
- 7. Amides 7 : Preparation 2) From Carboxylic Acids & Ammonium Carboxylates Direct reaction of carboxylic acids & ammonia yields ammonium salts Some ammonium salts of carboxylic acids can be dehydrated to the amide at high temperatures .This is generally a poor method of amide synthesis
- 8. Amides 8 : Reaction Amides are very unreactive compounds
- 9. Amides 9 : Reaction 1) Amides react with water or alcohol if the reaction is heated in presence of an acid
- 10. Amides 10 : Reaction 1) Amides react with water or alcohol if the reaction is heated in presence of an acid
- 11. Amides 11 : Reaction 1) Amides react with water or alcohol if the reaction is heated in presence of an acid Hydrolysis of Amides Heating an amide in concentrated aqueous acid or base causes hydrolysis
- 12. Amides 12 : Reaction 2) 1ry amides can be dehydrated to nitriles. Dehydrating reagents such as P2O5, POCl3, SOCl2 can be used.
- 13. Hofmann degradation 13 Conversion of primary amide to primary amine
- 14. Lactams 14 Cyclic amides are called lactams. The size of the lactam ring is designated by Greek letters in a way that is analogous to lactone nomenclature • γ-Lactams and δ-lactams often form spontaneously from γ- and δ-amino acids. • β-Lactams, however, are highly reactive; their strained four-membered rings open easily in the presence of nucleophilic reagents.
- 15. Nitriles 15 : Preparation A nitrile can be formed by reaction of an amide with phosphorous pentoxide or boiling acetic anhydride
- 16. Nitriles 16 : Reaction Hydrolysis of Nitriles
- 17. Nitriles 17 : Reaction Hydrolysis of Nitriles in acidic medium
- 18. Nitriles 18 : Reaction Hydrolysis of Nitriles in basic medium
- 19. Tautomerism 19 Tautomerism keto and enol forms of carbonyl compounds are constitutional isomers, but of a special type. Because they are easily interconverted by proton transfers in the presence of an acid or base. Interconvertible keto and enol forms are called tautomers, and their interconversion is called tautomerization enol form keto form C OH C C C O H The keto form is usually predominant with few exceptions.
- 20. 20 The keto form is usually predominant with few exceptions. 2,4-Pentanedione Enol form (76%) Tautomerism
- 21. Decarboxylation of Carboxylic Acids 21 Loss of CO2 from a carboxyl group = Decarboxylation • Carboxylic acids undergo thermal decarboxylation, when heated to a very high temperature and most carboxylic acids are resistant to moderate heat. • Exceptions are carboxylic acids that have a carbonyl group β to the carboxyl group (β-ketoacids undergo decarboxylation readily on mild heating).
- 22. Decarboxylation of Carboxylic Acids 22 Loss of CO2 from a carboxyl group = Decarboxylation • There are two reasons for this ease of decarboxylation: 1. When the acid itself decarboxylates, it can do so through a six-membered cyclic transition state: This reaction produces an enol (alkene-alcohol) directly and avoids an anionic intermediate. The enol then tautomerizes to a methyl ketone.
- 23. Decarboxylation of Carboxylic Acids 23 Loss of CO2 from a carboxyl group = Decarboxylation • There are two reasons for this ease of decarboxylation: 2. When the carboxylate anion decarboxylates, it forms a resonance-stabilized anion:. This type of anion is much more stable than simply RCH2:−, the anion that would have been produced by decarboxylation in the absence of a β-carbonyl group. It is known as an enolate.
- 24. Decarboxylation of Carboxylic Acids 24 Loss of CO2 from a carboxyl group = Decarboxylation • β-Dicarboxylic acids (1,3-dicarboxylic acids, also called malonic acids) decarboxylate readily for reasons similar to β-keto acids.
- 25. Decarboxylation of Carboxylic Acids 25 Loss of CO2 from a carboxyl group = Decarboxylation • COOH group at o-or p-position to OH group is easily replaced by Br or NO2 group
- 26. Nuclear substitution reactions 26 COOH is deactivator , thus m-directing group:
- 27. 27 Enolate formation • The pKa values for the α hydrogens of most simple aldehydes or ketones are of the order of 19–20 that are more acidic than hydrogen atoms of ethyne (pKa = 25), and ethene (pKa = 44) or of ethane (pKa = 50). • The carbonyl group is strongly electron withdrawing, and when a carbonyl compound loses an α proton, the anion that is produced, called an enolate, is stabilized by delocalization. The Acidity of the α Hydrogens of Carbonyl Compounds
- 28. 28 Carbonyl compounds bearing an α hydrogen can undergo halogen substitution at the α carbon in the presence of acid or base. The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon + H2O + Br - C CH3 O CH2Br Br2 / OH - C CH3 O CH3 + HBr C CH3 O CH2Br Br2 / H+ C CH3 O CH3
- 29. 29 Carbonyl compounds bearing an α hydrogen can undergo halogen substitution at the α carbon in the presence of acid or base. The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon (multiple halogenations can occur) Ex: Haloform reaction
- 30. 30 The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon
- 31. 31 The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon
- 32. 32 The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon α-Halo Carboxylic Acids: The Hell–Volhard–Zelinski Reaction Carboxylic acids bearing α hydrogen atoms react with bromine or chlorine in the presence of phosphorus (or a phosphorus halide) to give α- halo carboxylic acids through a reaction known as the Hell–Volhard–Zelinski (or HVZ) reaction. α-Iodo acyl chlorides can be obtained by using molecular iodine in a similar reaction.
- 33. 33 The Acidity of the α Hydrogens of Carbonyl Compounds Halogenation at the α Carbon
- 34. 34 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion Aldehydes or ketones containing α-H's, in the presence of alkali, give carbanion.
- 35. 35 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion Aldol condensation: Aldehydes or ketones containing α-H's, in the presence of alkali, give carbanion. The resulting carbanion attacks the carbonyl carbon of a second molecule. e.g., aldol condensation:
- 36. 36 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion a- Claisen-Schmidt reaction (crossed aldol): Condensation between benzaldehyde (which does not contain α-H) and aliphatic aldehyde or ketone (which contain α-H):
- 37. 37 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion a- Claisen-Schmidt reaction (crossed aldol): Condensation between benzaldehyde (which does not contain α-H) and aliphatic aldehyde or ketone (which contain α-H):
- 38. 38 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion b- Claisen reaction (for ester): Condensation of esters (containing at least 2 α-H's) in the presence of sodium ethoxide. N.B. NaOH causes hydrolysis to the ester.
- 39. 39 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion c- Crossed Claisen reaction (for ester): Aromatic aldehydes (or esters, which do not contain α-H) condense with an ester (which contains α-H) in the presence of sodium ethoxide.
- 40. 40 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion d- Knoevenagel condensation: Aromatic aldehydes (or other aldehydes or ketones) condense with esters having active α- H's in the presence of weak bases (amines).
- 41. 41 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion e- Perkin condensation: Aromatic aldehydes condense with a carboxylic acid anhydride to give α,b- unsaturated acid. The reaction is catalyzed by potassium salt of the carboxlic acid. NaOH should not be used, why ?? Benzaldehyde undergoes another reaction in the presence of NaOH. In all these aldol-type condensations, the principle involves abstraction of α-H by a base such as NaOH, NaOC2H5, amines, or salt of carboxlic acid.
- 42. 42 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion Cannizzaro reaction: Aldehydes that do not contain α-H, in the presence of concentrated NaOH, undergo selfoxidation-reduction reaction
- 43. 43 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion Crossed Cannizzaro reaction: A mixture of two aldehydes (with no α-H's), in the presence of concentrated sodium hydroxide, will give all possible products. If one aldehyde is formaldehyde, the reaction yields, exclusively sodium formate and the alcohol corresponding to the other aldehyde
- 44. 44 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion Crossed Cannizzaro reaction: A mixture of two aldehydes (with no α-H's), in the presence of concentrated sodium hydroxide, will give all possible products. If one aldehyde is formaldehyde, the reaction yields, exclusively sodium formate and the alcohol corresponding to the other aldehyde C Ar O H OH - Ar C O- H OH C Ar O H + Ar C O- H OH Ar C H H O- + Ar C O OH H+ - H+ Ar CH2OH Ar COO- Mechanism Not methyl alcohol and sodium benzoate are formed. Because the initial nucleophilic addition of hydroxide anion is faster on formaldehyde as there are no electron donating groups on it.
- 45. 45 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion intermolecular cannizzaro
- 46. 46 The Acidity of the α Hydrogens of Carbonyl Compounds Reactions of carbanion intermolecular cannizzaro
- 47. 48 a,b-Unsaturated Carbonyl compounds 1,4-addition b a R CH CH C O R' An a,b-unsaturated carbonyl compound has a carbon-carbon double bond in conjugation with a carbonyl group. The resonance structures show that the b-carbon, as well as the carbonyl carbon, carries a partial positive charge, while the carbonyl oxygen carries a partial negative charge. This can lead to 1,4-addition. + CH2 CH C O- H + CH2 CH C H O- CH2 CH C H O
- 48. 49 a,b-Unsaturated Carbonyl compounds 1,4-addition: A) Electrophilic 1,4-Addition So the Nucleophilic part (CN -, Cl -, OH -) is added to the b-carbon, which has a partial positive charge. keto form enol form Cl CH2 CH2 C O H CH2 CH C O Cl H H Cl - + CH2 CH C OH H CH2 CH C H OH+ H+ CH2 CH C H O
- 49. 50 a,b-Unsaturated Carbonyl compounds 1,4-addition: B) Nucleophilic 1,4-Addition Both carbonyl carbon and b carbon can be attacked by nucleophiles (CN-, NH2 -, OH-) to give (A) or (B). (B) (A) CH2 CN CH2 C O CH3 CH2 CH C OH CH3 CN CN - CH2 CH C O CH3 The mechanism in (A) is the same as shown in the formation of cyanohydrin, but in the formation of (B), it is 1,4-addition. Both reactions are possible. **A highly basic reagents such as, RMgX, attack C=O, while a weaker bases such as, CN- or R2NH, usually attack C=C. ** Aldehydes, being less hindered than ketones, usually undergo carbonyl attack.