Embed presentation
Download to read offline
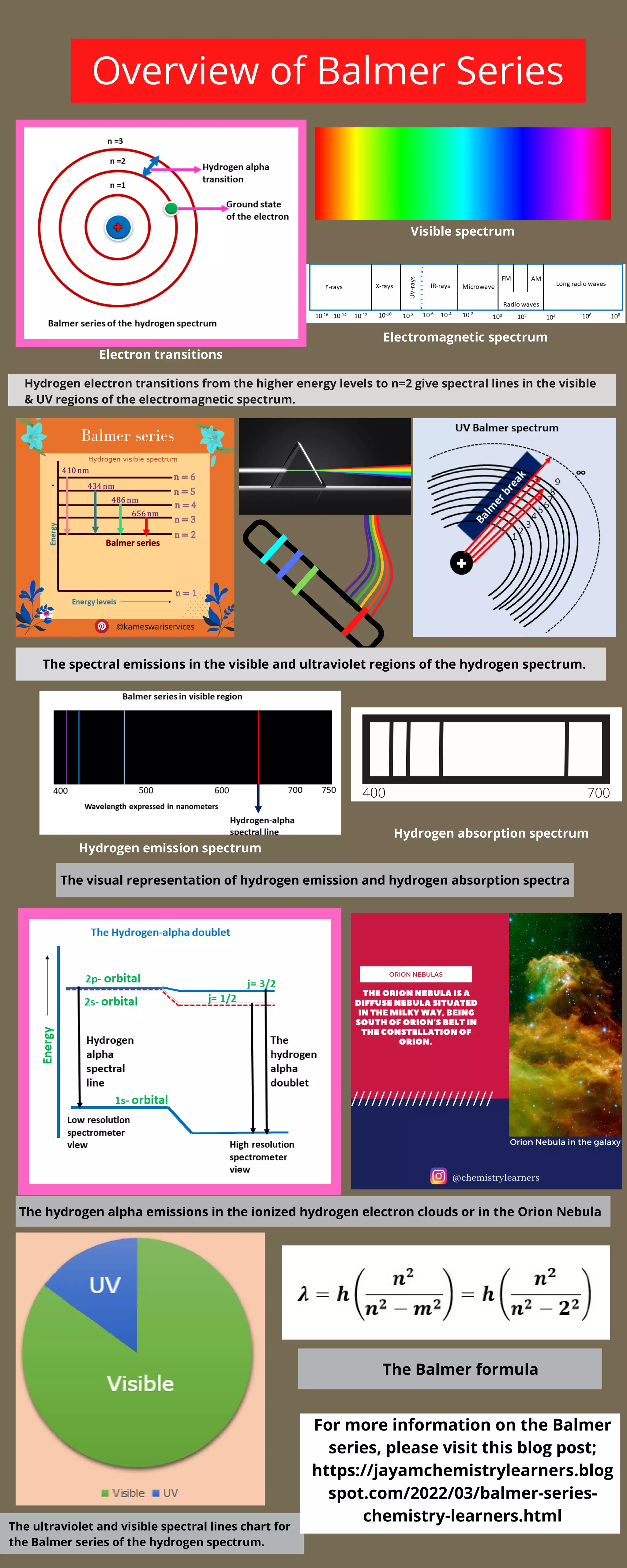
The document discusses the Balmer series in hydrogen's emission spectrum. Transitions of hydrogen's electrons from higher to the n=2 energy level produce spectral lines in the visible and UV regions of the electromagnetic spectrum. A chart shows the visible representation of hydrogen's emission and absorption spectra, including the hydrogen-alpha emissions seen in ionized hydrogen clouds like the Orion Nebula. The Balmer formula describes the ultraviolet and visible spectral lines of the Balmer series in hydrogen's spectrum.
