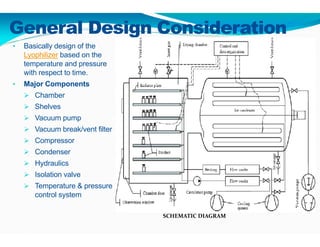
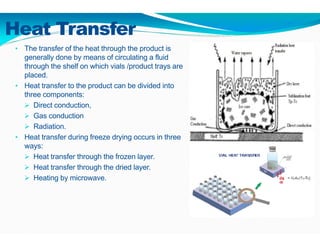
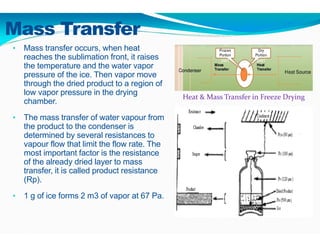
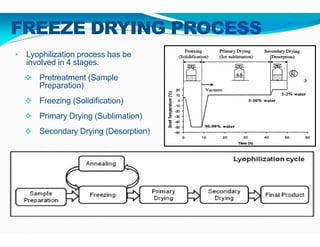
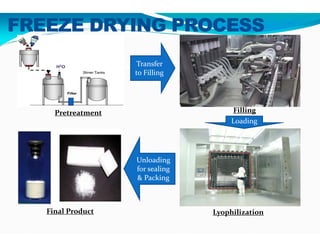
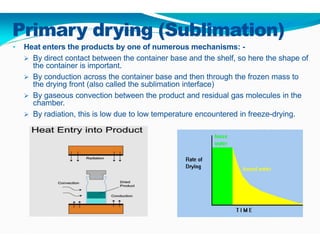
Freeze-drying, or lyophilization, is a preservation technique that involves cooling materials below certain temperatures and drying them in a vacuum to remove moisture. The process has been utilized since ancient times, becoming commercially developed during World War II for preserving medical supplies. It involves multiple stages including pretreatment, freezing, primary drying, and secondary drying, and finds applications in various industries such as pharmaceuticals and food processing.