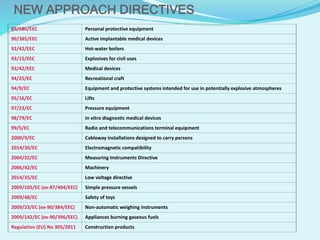
The document presents an overview of CE marking, which indicates a product's conformity to health, safety, and environmental standards in the European Economic Area. It outlines the necessity of CE marking for market entry, the benefits for manufacturers, and the process to get certified. The CE mark simplifies compliance by eliminating conflicting national regulations, enhancing product safety, and allowing for easier cross-border comparisons.