DRUGS USED IN TUBERCULOSIS
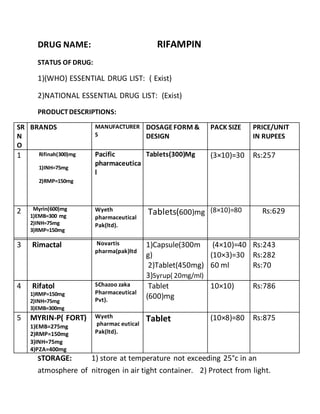
- 1. DRUG NAME: RIFAMPIN STATUS OF DRUG: 1)(WHO) ESSENTIAL DRUG LIST: ( Exist) 2)NATIONAL ESSENTIAL DRUG LIST: (Exist) PRODUCTDESCRIPTIONS: SR N O BRANDS MANUFACTURER S DOSAGEFORM & DESIGN PACK SIZE PRICE/UNIT IN RUPEES 1 Rifinah(300)mg 1)INH=75mg 2)RMP=150mg Pacific pharmaceutica l Tablets(300)Mg (3×10)=30 Rs:257 2 Myrin(600)mg 1)EMB=300 mg 2)INH=75mg 3)RMP=150mg Wyeth pharmaceutical Pak(ltd). Tablets(600)mg (8×10)=80 Rs:629 3 Rimactal Novartis pharma(pak)ltd 1)Capsule(300m g) 2)Tablet(450mg) 3)Syrup( 20mg/ml) (4×10)=40 (10×3)=30 60 ml Rs:243 Rs:282 Rs:70 4 Rifatol 1)RMP=150mg 2)INH=75mg 3)EMB=300mg SChazoo zaka Pharmaceutical Pvt). Tablet (600)mg 10×10) Rs:786 5 MYRIN-P( FORT) 1)EMB=275mg 2)RMP=150mg 3)INH=75mg 4)PZA=400mg Wyeth pharmac eutical Pak(ltd). Tablet (10×8)=80 Rs:875 STORAGE: 1) store at temperature not exceeding 25°c in an atmosphere of nitrogen in air tight container. 2) Protect from light.
- 2. CHEMISTRY OF THE PRODUCT CHEMICAL CLASS CHEMICAL STRUCTURE PHYSICAL PROPERTIES 4-methyl-1- piperazinaminylgroup, 1)Rifampicinis a reddish brown crystalline powder 2)slightly solubleinwater ,alcohol and in acetone and soluble inmethyl alcohol. CHEMICAL PROPERTIES 1)Melting point:(183–188) °C 2)1%SUSPENSION PH=4.5-6.5 3) Molecular mass: 822.94 g/mol Formula: C43H58N4O12 PHARMACOKINETICS : A)ABSORPTION: Orally administeredrifampicinresults inpeak plasmaconcentrations inabout ( 2 -4) hours.Rifampicinis easily absorbedfromthe gastrointestinal tract;its ester functional group is quickly hydrolyzed in the bile, and it is catalyzedby a high pH and substrate-specific enzymes calledesterases. After about six hours, almost all of the drug is deacetylated. Eveninthis deacetylatedform, rifampinis still apotent antibiotic;however, it canno longer be reabsorbedby the intestines andit is subsequently eliminatedfromthe body. . Whenrifampicinis takenwith a meal, peak blood concentrationfalls by 36%.
- 3. B)DISTRIBUTION: Distributionof the drug is high throughout the body, and reaches effective concentrations inmany organs and body fluids, including the CSF . Since the substance itself is red, this highdistributionis the reasonfor the orange-red color of the saliva, tears, sweat, urine, andfeces. C)ELIMINATION: Only about( 4-20)% of the administereddrug will be excretedunchanged throughthe urine, though urinary eliminationaccounts for only about 20% of the drug excretion.Rifampin is excretedmainly throughliver intobile,then undergoes enterohepatic recirculationwiththe bulk excretedas adeacylated metabolite infeces About 60% to85%. BIOAVAILABILITY %PROTEIN BINDING PLACENTAL BARRIER BBB SECRETION IN MILK VOLUME OF DISTRIBUTION TIME OF ONSET OF ACTION THERAPEUTIC SERUM LEVEL 90 – 95)% 80 -90)% rifampin has been reportedto cross the placental barrier and appear in cord blood, The combination of isoniazid and rifampicin for 1 year, with appropriate management increasesthe intracranial pressure. RIFAMPIN excreted during lactation, relatively safe in the minimal quantities ,nursing infants ingest through breast milk. Rifampin may harm a nursing baby. The apparent volume of distribution is1.6 L/Kg in adults and 1.1L/Kg in children. (2-3)h Rifampicin therapeutic levels( 7-10) Microgram/ml are attained after 2-3 hours.
- 4. Eliminationhalf life Site of metabolism Active metabolites Route of excretion Elimination half life increases with increasing doses and amounts to 2.5h,3.5h,and about 5h after single doses of 300mg,600mg and900mg respectively. Rifampin is metabolized in the liver. principal active metabolite is deacetylrifampicin Rifampicin metabolite deacetylrifampicin is excreted in the bile and also in the urine. Approximately 50% of the rifampicin dose is eliminated within 24 hours and (6 to 30)% of the drug is excreted unchanged in the urine, while 15% is excreted as active metabolite. Approximately( 43- 60)% of oral dose is excreted in the faeces. CLINICAL PHARMACOLOGY 1)THERAPEUTICCLASS: Antituberculosis 2)PHARMACOLOGICALCLASS: rifampinis a bactericidal antibioticdrug of the rifamycin group. It is a semisynthetic compound derived from Amycolatopsis mediterranei and Streptomyces mediterranei). 3)MECHANISM OF ACTION: RifampininhibitsDNA- dependentRNA polymerase activity in susceptible Mycobacterium tuberculosis organisms. Specifically,it interacts with bacterialRNA polymerase but does not inhibitthe mammalianenzyme.
- 5. 4)SPECTRUM: ( broad spectrum bactericidal) the most susceptible bacteria are: 1) Aerobic Gram-Negative Microorganisms Neisseria meningitidis Mycobacterium tuberculosis 2)Aerobic Gram-Positive Microorganisms Staphylococcus aureus (including Methicillin-Resistant S. aureus/MRSA) Staphylococcus epidermidis 3)Aerobic Gram-Negative Microorganisms Haemophilus influenzae 4)“Other” Microorganisms Mycobacterium leprae 5)INDICATIONS: Rifampicin is typically used to treat Mycobacterium infections, including tuberculosis and Hansen's disease (Leprosy). Rifampicin is used in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) in combination with fusidic acid , including in difficult to treat infections such as osteomyelitis and prosthetic joint infections.It is
- 6. also used in prophylactic therapy against Neisseria meningitidis (meningococcal) infection. It is also used to treat infection by Listeria species, Neisseria gonorrhoeae, Haemophilus influenzae and Legionella pneumophila 6A)CONTRAINDICATION : Rifampinis contraindicated in patients who are also receiving ritonavir-boosted saquinavir due to an increased risk of severe hepatocellular toxicity. Rifampinis contraindicated in patients who are also receiving atazanavir, darunavir, fosamprenavir,saquinavir, or tipranavir due to the potential of rifampin to substantially decrease plasma concentrations of these antiviral drugs, which may result in loss of antiviral efficacy and/or development of viral resistance. 6B)PRECAUTIONS: Rifampin is not recommended for intermittent therapy; the patient should be cautioned against intentional or accidental interruption of the daily dosage regimen since rare renal hypersensitivity reactions have been reported when therapy was resumed in such cases. Rifampinhas enzyme induction properties that can enhance the metabolism of endogenous substrates including adrenal hormones, thyroid hormones, and vitamin D.
- 7. 7)POSSIBLE SIDE EFFECTS: Headache,drowsiness,dizziness,numbness,thrombocytopenia, Eosinophilia,leukopenia,hemolyticanemia,epigastric distress, Nausea,vomiting,anorexia,skin allergy. 8) ADVERSE REACTIONS: A)Hepatic Transient abnormalities inliver functiontests (e.g., elevations inserum bilirubin, BSP, alkaline phosphatase, serumtransaminases) have beenobserved. B)Hematologic Rare reports of disseminatedintravascular coagulationhave been observed. C)Central Nervous System :Psychoses have beenrarely reported. D)Endocrine Rare reports of adrenal insufficiency inpatients with compromisedadrenal functionhave beenobserved. E)Renal Acute tubular necrosis F)Hypersensitivity Reactions; Erythemamultiforme including Stevens- Johnson Syndrome, toxic epidermal necrolysis,vasculitis. Anaphylaxis has been reportedrarely. G)Gastrointestinal : Pancreatitis H)Hypersensitivity Reactions:( Stevens-Johnson syndrome)
- 8. Dosage and administration: Sr no indications Route of administration Neonates/infants mg/kg/day frequency Child mg/kg/day frequency Adult mg/kg/day Duration of therapy 1 Rifampicin is recommended during the initial initial intensive phaseof short course Treatment of tuberculosis which usually lasts two months on a daily basis. Rifampicin and INH for initial intensive phase. Oralroute 5mg/kg daily in two divided doses. 60 ml syrup (20mg/ml) 10mg/kg Daily in two divided doses. 600mg- 1200mg daily in two divided doses. 6-8 months therapy With RMP+INH +PZA AND EMB) Administrationguidelines: A)for oral route: To ensure optimum absorption of oral rifampicinshould preferably be taken on an empty stomach,at least ½ h before a meal.syrup should be well shaken before use. B)For I/V Route:
- 9. PREPARATIONof Solution for IV Infusion : Reconstitute the lyophilizedpowder by transferring 10 mL of sterile water for injection to a vialcontaining600 mg of rifampin for injection.Swirl vial gently to completely dissolve the antibiotic.The reconstituted solutioncontains60 mg rifampin per mL and is stable at room temperature for 24 hours. Prior to administration,withdraw from the reconstituted solutiona volume equivalentto the amount of rifampin calculatedto be administered and add to 500 mL of infusion medium. Mixwell and infuse at a rate allowingfor complete infusion within 3 hours. Alternatively,the amount of rifampin calculatedto be administered may be addedto 100 mL of infusion medium and infused in 30 minutes. STABILITY: Dilutionsin dextrose 5% for injection(D5W) are stable at room temperature for up to 4 hours and should be prepared and used within this time. Precipitationof rifampin from the infusion solutionmay occur beyond this time. Dilutionsin normal saline are stable at room temperature for up to 24 hours and should be prepared and used within this time. Other infusion solutionsare not recommended.
- 10. FOOD-DRUG INTERACTION: Food delays and reduces the absorption of rifampicin from the gut: Clinical evidence:The absorptionof a single 10-mg/kg dose of rifampicin was reducedwhen it was givento 6 healthy subjects withastandard Indian breakfast (125 g wheat, 10 g visible fat, 350 g vegetables). The AUC after 8 hours was reducedby 26% and the peak plasma levels were prolonged(from 11.84 micrograms/mL at 2 hours to 8.35 micrograms/mL at 4 hours) and reducedby about 30%.1 Inanother study, a high-fat breakfast reduced the maximum serumlevel of rifampicin600 mg by 36% and delayedthe absorption, but the AUC was not significantly altered. Mechanism: Not understood. Importance and management:Rifampicinshouldbe takenon an empty stomach (at least 30 minutes before ameal, or 2 hours after a meal) to ensure rapid and complete absorption.
- 11. DRUG-DRUG INTERACTIONS; interactions Significance level outcome Mecha- nism Recomme- ndation ACE-inhibitors (enalapril) +Rifampin Onset=rapid Severity=moderate Documentation=possi ble Phamacologi cal effects of enalapril may decrease. unknow n Consider monitoring of B.P in patients that are receiving combination therapy.if B.P increases alternative antihypertensiv e therapy is recommended. Aminophylline+ rifampin Onset=delay Severity=moderate Document=established Phamacologica l levels of aminophylline may decrease with increase exacerbation of pulmonary symptoms. Increased hepatic metabolis m via enzyme induction of rifampin. Addition or deletion of rifampin therapy. Beta blockers(propranolol) +rifampin Onset=delay Severity=moderate Document=probable Beta blocker effects may be reduced via rifampin. Increased hepatic metabolis m from enzyme induction via rifampin. Consider monitoring of B.P in patients that are receiving combination therapy.if B.P increases alternative antihypertensiv e therapy is recommended. BARBITURATES( phenobarbitol,pentobarbital, Amobarbitol,Butabarbitol)+ rifampin Onset=delay Severity=minim Document=possible May result in increase rate of metabolism of barbiturates and reduce its pharmacological actions. Rifampin may stimulate microsomal enzyme and increase degradatio n of barbiturate. Barbiturate plasma level is monitored and dosage mey need to increase.
- 12. interaction Significant level outcome mechanism recomendation Contraceptives+ rifampin Onset= delay Severity=moderate Document=established May decrease oral contraceptive efficacy and also may increase incidences’ of menstrual abnormalities. Rifampin induces hepatic microsomal enzymes that may result in more rapid elimination of estrogenic and progestational components of oral contraceptives. Advise patient to use an additional form of contraceptive in rifampin therapy. Quinine derivatives+rifampin Onset=delay Severity=moderate Document=establish May increase metabolism of quinine derivatives that may be associated in reduction in therapeutic effects. Rifampin is potent inducer of hepatic microsomal enzymes that may increase the hepatic clearance of quinine derivatives. Advise patient to increase dosage of quinine derivaties to maintain therapeutic effects. Drug/Laboratory Interactions 1)Cross-reactivity and false-positive urine screening tests for opiateshave been reported in patientsreceiving rifampin when using the KIMS (Kinetic Interaction of Microparticlesin Solution)method (e.g., Abuscreen On Line opiates assay; Roche Diagnostic Systems). Confirmatory tests, such as gas chromatography/ mass spectrometry, will distinguish rifampin from opiates. 2)Therapeutic levelsof rifampin have been shown to inhibit standard microbiologicalassays for serum folate and vitamin B12. Thus, alternate assay methods shouldbe considered.
- 13. Transient abnormalitiesin liver function tests (e.g., elevationin serum bilirubin,alkalinephosphatase,and serum transaminases) and reduced biliaryexcretion of contrast media used for visualization ofthe gallbladderhave also been observed. Therefore, these tests should be performed before the morning dose of rifampin. Rifampin in pregnancy: FDA pregnancy category C. This medication may be harmful to an unborn baby. Rifampinhas beenshown to be teratogenic inrodents givenoral doses of rifampin 15 to 25 times the human dose. Althoughrifampin has beenreportedtocross the placental barrier andappear in cord blood, the effect of rifampin, alone or in combinationwithother antituberculosis drugs on the human fetus is not known. Neonates of rifampin-treatedmothersshouldbe carefully observedfor any evidence of adverse effects. Isolatedcases of fetal malformations have been reported;however, there are noadequate and well- controlledstudiesinpregnant women. Rifampinshould be usedduring pregnancy only if the potential benefit justifies the potential risk tothe fetus. Pregnancy-Non-Teratogenic Effects: When administeredduring the last fewweeks of pregnancy, rifampincan cause post-natal hemorrhages inthe mother and infant for which treatment with vitaminK may be indicated. Nursing Mothers: Because of the potential for tumorigenicity shown for rifampinin animal studies, adecisionshouldbe made whether to discontinue nursing or discontinue the drug, taking intoaccount the importance of the drug to the mother.
- 14. Toxicology: (OVERDOSAGE) Signs and Symptoms: Nausea, vomiting, abdominal pain, pruritus, headache and increasing lethargy will probably occur within a short time after ingestion; unconsciousness may occur when there is severe hepatic disease. Transient increases in liver enzymes and/or bilirubin may occur. Brownish-red or orange discoloration of the skin, urine, sweat, saliva, tears and feces will occur, and its intensity is proportional to the amount ingested.Liver enlargement, possibly with tenderness, can develop within a few hours after severe overdosage; bilirubin levels may increase and jaundice may develop rapidly. A direct effect upon the hematopoietic system, electrolyte levels, or acid-base balance is unlikely. Hypotension, sinus tachycardia, ventricular arrhythmias, seizures and cardiac arrest were reported in some fatal cases. Acute Toxicity: The LD50 of rifampin is approximately 885 mg/kg in the mouse, and 2120 mg/kg in the rabbit.The minimum acute lethal or toxic dose is not well established. However, nonfatal acute overdoses inadults have been reportedwithdoses ranging from9 to 12 gm rifampin. Fatal acute overdoses in adults have beenreportedwithdoses ranging from14- 16gm. Treatment:The airway should be secured and adequate respiratory exchange established. Since nausea and vomiting are likely to be present, gastric lavage within the first 2 to 3 hours after ingestion is probably preferable to induction of emesis. Following evacuation of the gastric contents, the instillation of activated charcoal slurry into the stomach may help absorb any remaining drug from the gastrointestinal tract. Antiemetic medication may be required to control severe nausea and vomiting.
- 15. REFRENCES: 1)Basic and clinical pharmacology by katzung(11th edition) 2) http:/www.medincell.com 2) drugs interactions facts via Davis.Tatro(4th edition) 3) http:/www.medincell.com 4) Pharmacology (Lippincott's Illustrated Reviews Series)[Richard A. Harvey PhD, Michelle A Clark PhD, Richard Finkel PharmD. 5) www.fda.gov/Food/default.htm . 6) www.pharmaguideindia.com/.
