lecture 2 slides.pptxlecture 2 slides sdfswssdsdsdsdsdsdsdsd.pptxlecture 2 slides.pptxlecture 2 slides.pptxlecture 2 slides.pptxlecture 2 slides.pptx
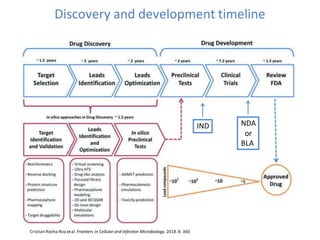
- 1. Discovery and development timeline Cristian Rocha-Roa etal. Frontiers in Cellular and Infection Microbiology. 2018.8. 360 IND NDA or BLA
- 2. Comments on previous slide • Pharmacophore is the 3D orientationof the functional groups of a molecule that interacts with target protein • Druggability is a term used in drug discovery to describe a biological target that is known to or is predicted to bind with high affinity to a drug. Furthermore,by definition, the binding of the drug to a druggable target must alter the function of the target with a therapeutic benefit to the patient.
- 3. CMC (Chemistry Manufacturing & Controls) componentsof NDA (New Drug Application)
- 4. Process chemistry • Process development begins with drug discovery • Discovery chemists – Prepares many compounds from a common intermediate. – convenient routes to preparesimilar compounds • Development chemists – Develops and optimizes the processes to prepareone compound with minimum impurities
- 5. Formulation development • Understanding physicochemical properties of a compound is essential in rational formulation and process development. – Permeability – Ionization Constant (pKa) – Solubility – Stability (hydrolytic, oxidative, photolytic) – Solid-state properties
- 6. Formulation Development - Challenges • Approaches to overcome solubility/dissolution-limited bioavailability Chemical modification (Salt or prodrug) Particle size reduction (micronizationand nanonization) Incorporation of surfactants Solid dispersions Complexation (β-CD)
- 7. Solid-State Form and Particle Size Salt Screen Compound that can be ionized – Solubility/Dissolution Rate – Stability (chemical and physical) – Processing – Bioavailability/ProductPerformance Form and Polymorph Screen To identify and characterizedifferent forms – To mitigate risks associatedwith form definition, such as variable product performance, regulatoryissues and manufacturing. – Stability (chemical and physical) – Solubility/Dissolution Rate – Processing – Bioavailability/ProductPerformance. Particle Size – Drug particle size may impact dissolution rate, bioavailability, content uniformity, stability,flow characteristics. – Establishing impact of particle size on dissolution and PK performance is essential, especially for BCS class II and IV.
- 8. Preclinical studies • Use animals and/or cells or tissues. • Give informationabout candidate’s – Pharmacodynamics – Pharmacokinetics – Toxicology • Information from non-clinical testing is used in planning clinical trials in humans – startingdose should be – range of does to be tested – clinicalsigns of expected side effects
- 9. Clinical Trials • Clinical trials of drugs provide information about: Whether the drug has the effect it is supposed to have. How much of the drug to give to a patient and how often. What side effects are associated with the drug and how they can best be managed. How a drug is broken down in the body, and how long it stays in the body. Which foods, drinks, or other drugs can be used at the same time or should be avoided. Clinical trial results allow the FDA to make decisions about whether or not a drug should be approved for marketing. Courtesyof David Kiere ( Acting Laboratory Chief, Branch I of the CDER of the US FDA)
- 10. Phase 1 • Healthy volunteers. • The goal here is to determine what the drug's most frequentside effects(DRUG SAFETY)are and, often, how the drug is metabolized and excreted. • The number of subjects typicallyranges from 20 to 80 • Are there any unacceptabletoxicities? • Takes months • ~70 % move on to phase 2.
- 11. Phase 2 • Is the drug effective? • Does the drug safely work in people who have a certain disease or condition. • Could be few dozen to about 300 patients. • Months- years • ~33 % move on to phase 3
- 12. Phase 3 • More information about safety and efficacy – different populations? – different dosages? – combinations with other drugs? • The number of subjects usually ranges from several hundred to about 3,000 people. • Usually double-blind • Years to decades • ~25-30 % move on to the market
- 13. Common Problems-clinical trials • Safety issues or failure to demonstrate a drug's effectiveness. – A sponsor may need to conduct additional studies-perhaps studies of more people, different types of people or for a longer period of time. • Scale up problems: the drug made for clinical testing is not the same as the final manufactured drug.
- 14. Cont’d • Manufacturing issues are also among the reasons that approval may be delayed or denied. – Drugs must be manufactured in accordance with standards called GMP – FDA inspects manufacturing facilities before a drug can be approved. – If a facility isn't ready for inspection, approval can be delayed. Any manufacturing deficiencies found need to be corrected before approval.
- 15. Cost of development 2 1 The calculations were based on 63 drugs developed by publicly traded U.S.-based biopharma companies.
- 16. 16 Considerations in the design of drug product A. Biopharmaceutic Considerations The essential elements of the biopharmaceutical considerations in drug product design include: • (1) studies done to decide the physicochemical nature of the drug to be used, for example, salt and particle size; • (2) the timing of these studies in relation to the preclinical studies with the drug; • (3) the determination of the solubility and dissolution characteristics; • (4) the evaluation of drug absorption and physiological disposition studies; and • (5) the design and evaluation of the final drug formulation.
- 17. 17 Goal and barriers • Goal: The drug product must effectivelydeliver the active drug at an appropriate rate and amount to the target receptorsite so that the intendedtherapeuticeffectis achieved. • Barriers: To achieve this goal, the drug – must traverse the requiredbiologicalmembrane barriers, – escape widespreaddistributionto unwantedareas, – endure metabolicattack,and – cause an alterationof cellular function.
- 18. Reaching a middle ground • The finished drug product is a compromise of various factors, including – therapeuticobjectives, – pharmacokinetics, – physicaland chemical properties, – manufacturingcost,and – patient acceptance. • Mostimportant, the finished drug productshould meet the therapeutic objective by delivering the drug with maximum bioavailability and minimum adverse effects 18
- 19. 19 B. Pharmacodynamic Considerations Pharmacodynamics is the study of the effect of a drug in the body and its mechanism of action • An oral drug used to treat an acute illness is generally formulated to release the drug rapidly, allowing for quick absorption and rapid onset. • If more rapid drug absorption is desired, then an injectable drug formulation might be formulated.
- 20. • In the case of nitroglycerin, which is highly metabolized if swallowed, a sublingual tablet formulation allows for rapid absorption of the drug from the buccal area!! (sublingual area) of the mouth for the treatment of angina pectoris. 20
- 21. • For the treatment of certain diseases, such as hypertension, chronic pain, etc, an extended- or controlled-release dosage form is preferred. • The extended-release dosage form releases the drug slowly, thereby controlling the rate of drug absorption and allowing for more constant plasma drug concentrations. 21
- 22. • In some cases, an immediate-drug-release component is included in the extended-release dosage form to allow for both rapid onset followed by a slower sustained release of the drug, for example, zolpidem tartrate extended-release tablets (Ambien® CR tablets). 28 Qiang Fu. et al. PowderTechnology,Volume 301,2016
- 23. C. Drug Substance Considerations • Physicochemicalproperties of DS discussed in table 15-1 are major factors that are controlled or modified by the formulator. • Many approaches are used to address these properties 23
- 24. D. Pharmacokinetics of the Drug • Clinical failures of about 50% of the InvestigationalNew Drug (IND) filings are attributed to their inadequate ADME attributes. • Therefore, pharmaceutical industry is searching for ever more effective means to minimize this problem. • Approaches to solve the above problem? • Role of polymorphism. Which polymorphism? Genetic polymorphism.(will be covered) 24
- 25. 25 E. Bioavailability of the Drug • pharmacologic response is generally related to the concentration of drug at its site of action, • the availability of a drug from a dosage form is a critical element of a drug product’s clinical efficacy. • However, most bioavailabilitystudies involve the determination of drug concentration mainly in the plasma since it is rather difficult to measure the concentration at the site of action. • Need to consider stability in GIT and presystemicelimination
- 26. 26 F. Dose Considerations • Some patients experience unique differences from the regular adult population in pharmacokinetic parameters due to – differences in metabolic background, – renal clearance, – weight, – volume of distribution, – age, and – disease stage (eg, liver impairment, renal impairment), • Consequently,require individualized dosing. • Therefore, the drug product must usually be available in several dose strengths to allow for individualized dosing and possibly dose titration. • Some tablets are also scored for breaking, to potentially allow the administration of fractional tablet doses.
- 27. • The size and the shape of a solid oral drug product are designed for easy swallowing. • For oral dosage forms, if the recommended dose is large (1 g or more), then the patient may have difficulty in swallowing the drug product. • many patients may find a capsule-shaped tablet (caplet) easier to swallow than a large round tablet 27
- 28. 28 G. Patient Considerations • The drug product and therapeutic regimen must be acceptable to the patient. • Poor patient compliance may result from poor product attributes, such as – difficulty in swallowing, – disagreeable odor, – bitter medicine taste, or – frequent and/or unusual dosage requirements.