Novel drugs approved by fda in 2021
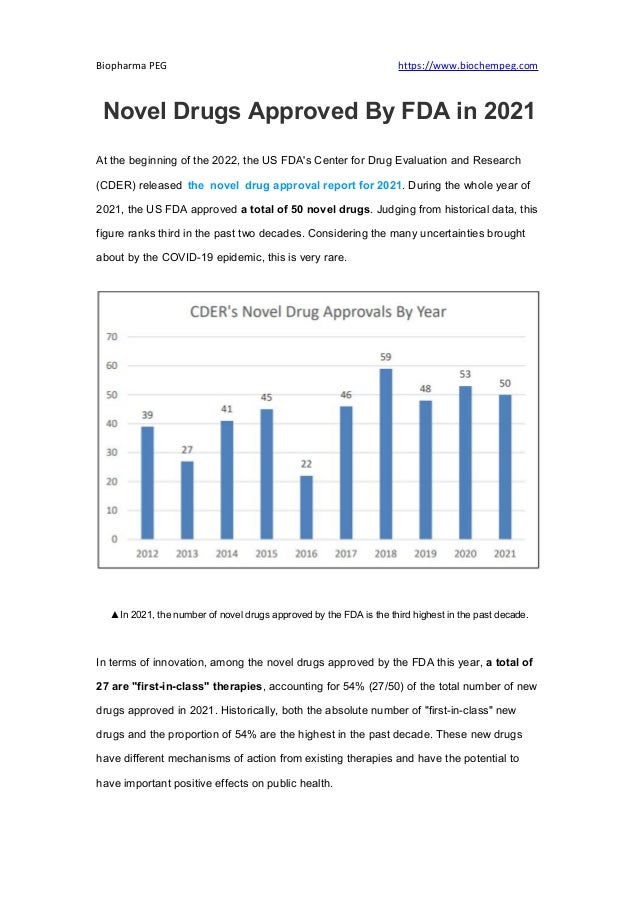
- 1. Biopharma PEG https://www.biochempeg.com Novel Drugs Approved By FDA in 2021 At the beginning of the 2022, the US FDA's Center for Drug Evaluation and Research (CDER) released the novel drug approval report for 2021. During the whole year of 2021, the US FDA approved a total of 50 novel drugs. Judging from historical data, this figure ranks third in the past two decades. Considering the many uncertainties brought about by the COVID-19 epidemic, this is very rare. ▲In 2021, the number of novel drugs approved by the FDA is the third highest in the past decade. In terms of innovation, among the novel drugs approved by the FDA this year, a total of 27 are "first-in-class" therapies, accounting for 54% (27/50) of the total number of new drugs approved in 2021. Historically, both the absolute number of "first-in-class" new drugs and the proportion of 54% are the highest in the past decade. These new drugs have different mechanisms of action from existing therapies and have the potential to have important positive effects on public health.
- 2. Biopharma PEG https://www.biochempeg.com ▲"first-in-class" therapies, accounting for 54% of the total number of new drugs approved in 2021. There were 50 novel drugs approved by the FDA in 2021. What are their approval dates, trade names, active ingredients and indications? Let's have a look at the below table.
- 4. Biopharma PEG https://www.biochempeg.com ▲CDER’s Novel Approvals of 2021 (in alphabetical order) Below we select some new drugs that have achieved breakthroughs in "zero to one" for a brief introduction (in alphabetical order).
- 5. Biopharma PEG https://www.biochempeg.com Aduhelm: FDA's first new therapy for Alzheimer's disease in nearly 20 years From the submission of the application to the approval half a year later, the discussion about Aduhelm in the field has not stopped. On a positive side, its approval is expected to inspire the field as a whole, spur investment in innovative treatments, and encourage more innovations to come. "Aducanumab is only the first of several Alzheimer's drugs expected to benefit patients over the next 5-10 years," said Dr. Howard Fillit, Founding Executive Director and Chief Scientific Officer, Alzheimer's Drug Discovery Foundation (ADDF). "The rich R&D pipeline for Alzheimer's disease, along with the availability of more biomarkers and other important research tools, means that clinical trials currently underway are more rigorous and more promising." Cabenuva: the first long-acting HIV injection therapy Cocktail therapy for HIV infection is one of the most important advances in medicine in the past 25 years. As long as patients stick to their daily medication, their longevity is not
- 6. Biopharma PEG https://www.biochempeg.com significantly different from that of healthy people. But daily medication is a burden for many patients. Cabenuva is the first full injection regimen approved by FDA for HIV-infected adults, requiring only one treatment per month, greatly reducing the pressure on patients to take their daily medication. Cabenuva is an intramuscular long-acting antiviral therapy consisting of Rilpivirine and Cabotegravir. Rilpivirine is an oral non-nucleoside reverse transcriptase inhibitor. Cabotegravir is an integrase inhibitor that inhibits the integration of viral DNA into the genome of human immune cells. Leqvio: The first siRNA therapy to lower 'bad cholesterol' Leqvio is a "first-in-class" siRNA therapy that binds to the mRNA encoding the PCSK9 protein, reduces its levels through RNA interference, and prevents the liver from producing PCSK9 protein. When their levels are lowered, more LDL receptors can return to the surface of liver cells, clearing LDL from the blood.
- 7. Biopharma PEG https://www.biochempeg.com This therapy provides long-lasting control with only two treatments per year after the initial injection and the third month of injection therapy. This is an important step towards RNA interference therapy for diseases affecting a wide range of populations, and long-acting therapy is also a future direction of RNA interference therapy. Lumakras: Breaking the Undruggableness of KRAS Mutations in the KRAS gene are one of the most common mutations in cancer and are themselves a well-known "undruggable" target. In non-small cell lung cancer, about 25% of patients have KRAS mutations, of which 13% have KRAS G12C mutations. Lumakras is a covalent inhibitor that specifically targets KRAS G12C mutants. It binds to KRAS G12C mutants and locks KRAS in an inactive state, thereby irreversibly inhibiting KRAS activity. Dr. Richard Pazdur, director of the FDA Oncology Center of Excellence and acting director of the Office of Oncology Diseases in the FDA's Center for Drug Evaluation and Research, pointed out that KRAS mutations have been difficult to target by drugs and represent a real unmet therapeutic need. The approval of Lumakras represents another major advance in personalized therapy.
- 8. Biopharma PEG https://www.biochempeg.com Pepaxto: The First Approved Peptide-drug Conjugate Anticancer Drug Pepaxto is a "first-in-class" peptide-drug conjugate that couples an alkylating agent to an aminopeptidase-targeting peptide. Due to its lipophilicity, Pepaxto is rapidly taken up by multiple myeloma cells, where it is rapidly hydrolyzed by peptidases to release the hydrophilic alkylating agent. In in vitro experiments, Pepaxto was 50 times more potent in killing multiple myeloma cells than the alkylating agent it carried due to its ability to increase the intracellular concentration of the alkylating agent. The molecular weight of peptide drugs is between small molecule drugs and biological products. Compared with biological products, peptides have many advantages: their design is simpler, they can interact with underexplored targets, and they have lower immunity. Originality and better tissue penetration. In addition to being biologically active, peptides are excellent at delivering drugs to specific targets. Rybrevant: the first approved bispecific antibody targeting different tumor antigens Rybrevant is a humanized EGFR/MET bispecific antibody. It has multiple anticancer mechanisms, not only blocking EGFR and MET-mediated signaling, but also directing
- 9. Biopharma PEG https://www.biochempeg.com immune cells to target tumors harboring activating and resistant EGFR/MET mutations and amplifications. This approval represents a milestone for the field of bispecific antibody development. This is the first FDA approval of a bispecific antibody therapy targeting different tumor antigens. Tezspire: Benefiting Severe Asthma sufferers Asthma is a highly heterogeneous disease that affects approximately 340 million people worldwide. Among them, about 10% of asthma patients have severe asthma. Many people with severe asthma remain uncontrolled, despite their access to therapies such as inhaled asthma medications. And because of the complexity of severe asthma itself, the pathogenesis behind many patients is unknown. Tezspire is a monoclonal antibody therapy targeting anti-thymic stromal lymphopoietin (TSLP). TSLP is an epithelial cytokine that sits at the apex of multiple inflammatory
- 10. Biopharma PEG https://www.biochempeg.com cascades that initiate hypersensitivity, eosinophilic, and other types of hyperimmune responses to airway inflammation associated with severe asthma. Many patients with severe asthma still experience frequent exacerbations, severely reducing quality of life and increasing the risk of hospitalization, said the principal investigator of the new drug's clinical trial. Tezspire offers a much-needed new treatment option for people with severe asthma who cannot control their symptoms. Verquvo: another first for heart failure patients Verquvo is the first innovative drug approved by the US FDA in 2021 and the first soluble guanylate cyclase (sGC) agonist to treat patients with exacerbating chronic heart failure. sGCs are important for both vascular and cardiac function, however, in patients with heart failure, sGCs are not fully activated, resulting in abnormal myocardial and vascular function. Verquvo restores the function of a key signaling pathway (NO-sGC-cGMP) by activating sGC. Welireg: The first anti-cancer therapy spawned by the Nobel Prize signaling pathway
- 11. Biopharma PEG https://www.biochempeg.com Von Hippel–Lindau disease is a rare genetic disorder that abnormally activates hypoxia-inducible factor (HIF-2α) in cancer patients. The accumulation of the latter in patients can lead to the formation of benign and malignant tumors, such as renal cell carcinoma, central nervous system hemangioblastoma, or pancreatic neuroendocrine tumors. The HIF-2α pathway related research won the Nobel Prize in Physiology or Medicine in 2019. As a potent and selective novel oral HIF-2α inhibitor, Welireg is an innovative therapy transformed from the Nobel Prize discovery, which once again emphasizes how science can benefit human health. Zynlonta: First Approved Antibody Drug Conjugate Targeting CD19 Zynlonta is the first FDA-approved antibody-drug conjugate targeting CD19. It couples a humanized anti-CD19 monoclonal antibody to a unique cytotoxin called a pyrrolobenzodiazepine (PBD) dimer. CD19 is a specific antigen expressed on the surface of B cells and is the target of a variety of CAR-T therapies. After PBD enters the cell, it irreversibly binds to DNA, efficiently cross-linking between the two strands of the DNA double helix, preventing the separation of the DNA strands, and eventually leading to the death of cancer cells.
- 12. Biopharma PEG https://www.biochempeg.com Biopharma PEG supplies non-cleavable ADC linker to support ADC drug Zynlonta's research. The CDER report introduces not only innovative drugs approved in 2021, but also a number of other notable approvals. In 2022, we expect to see more innovative therapies approved, bringing new treatment options to patients around the world. References: [1] Advancing Health Through Innovation: New Drug Therapy Approvals, 2021. [2] Mullard., (2022). 2021 FDA approvals. Nature Reviews Drug Discovery [3] 2021's NDA list includes some extraordinary accomplishments in year #2 of the pandemic Related article: [1]FDA Approves Besremi (ropeginterferon alfa-2b-njft) for Treating Polycythemia Vera [2]FDA Approves Tivdak - First Tissue Factor (TF)-Targeted Antibody Conjugate Drug (ADC) [3]FDA Approval of ZYNLONTA™ (loncastuximab tesirine-lpyl) - First CD19-targeted ADC