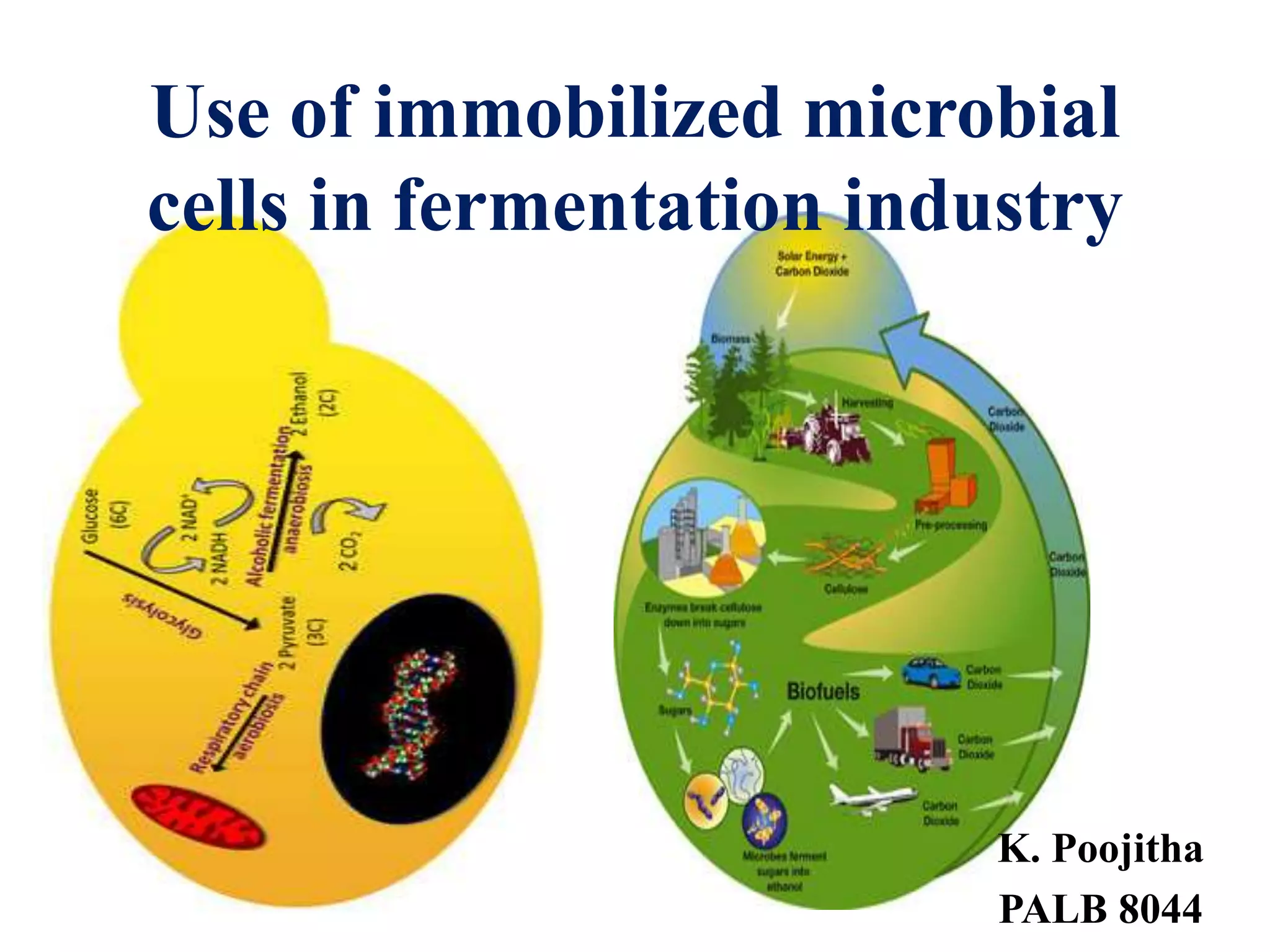
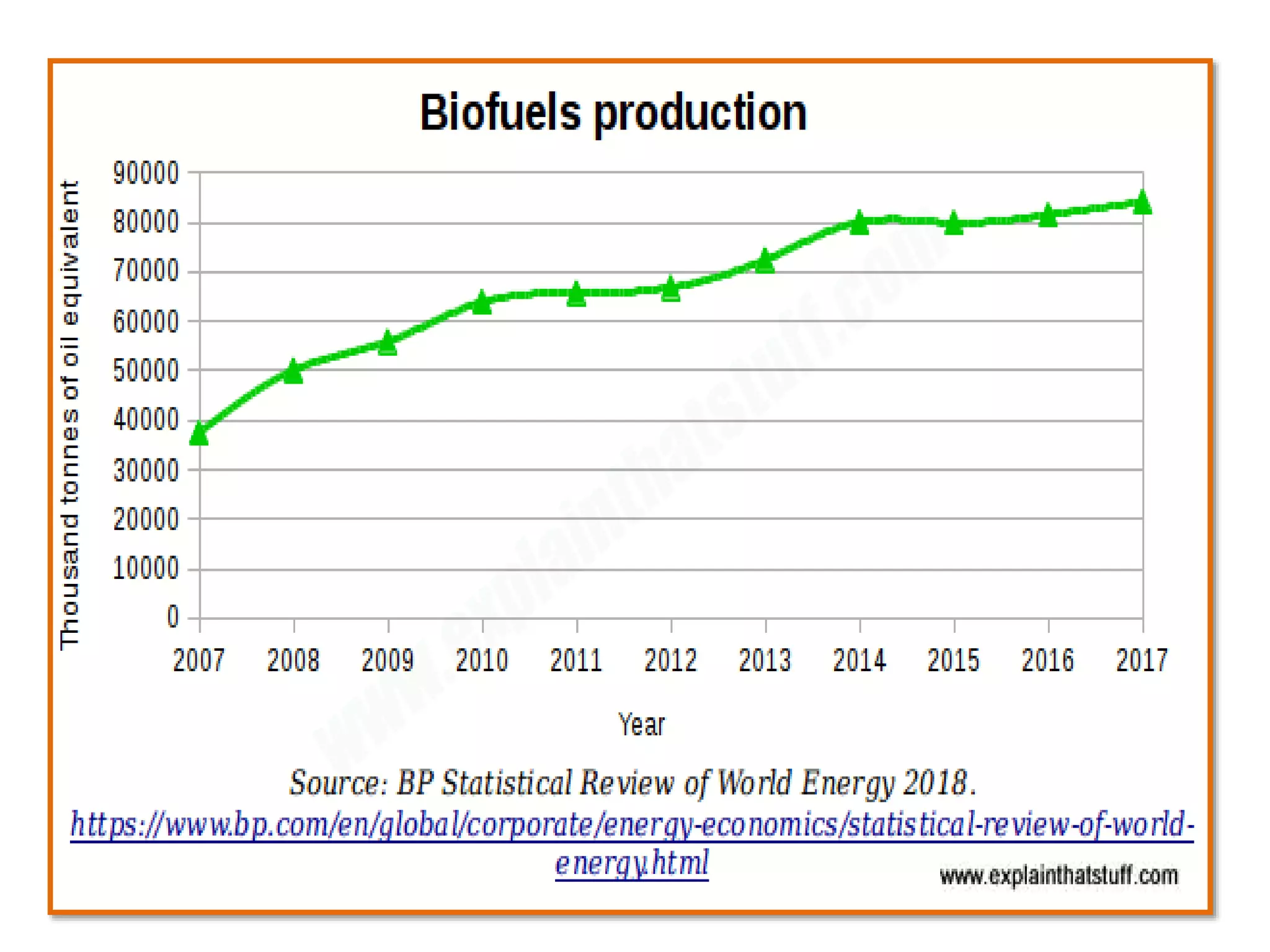
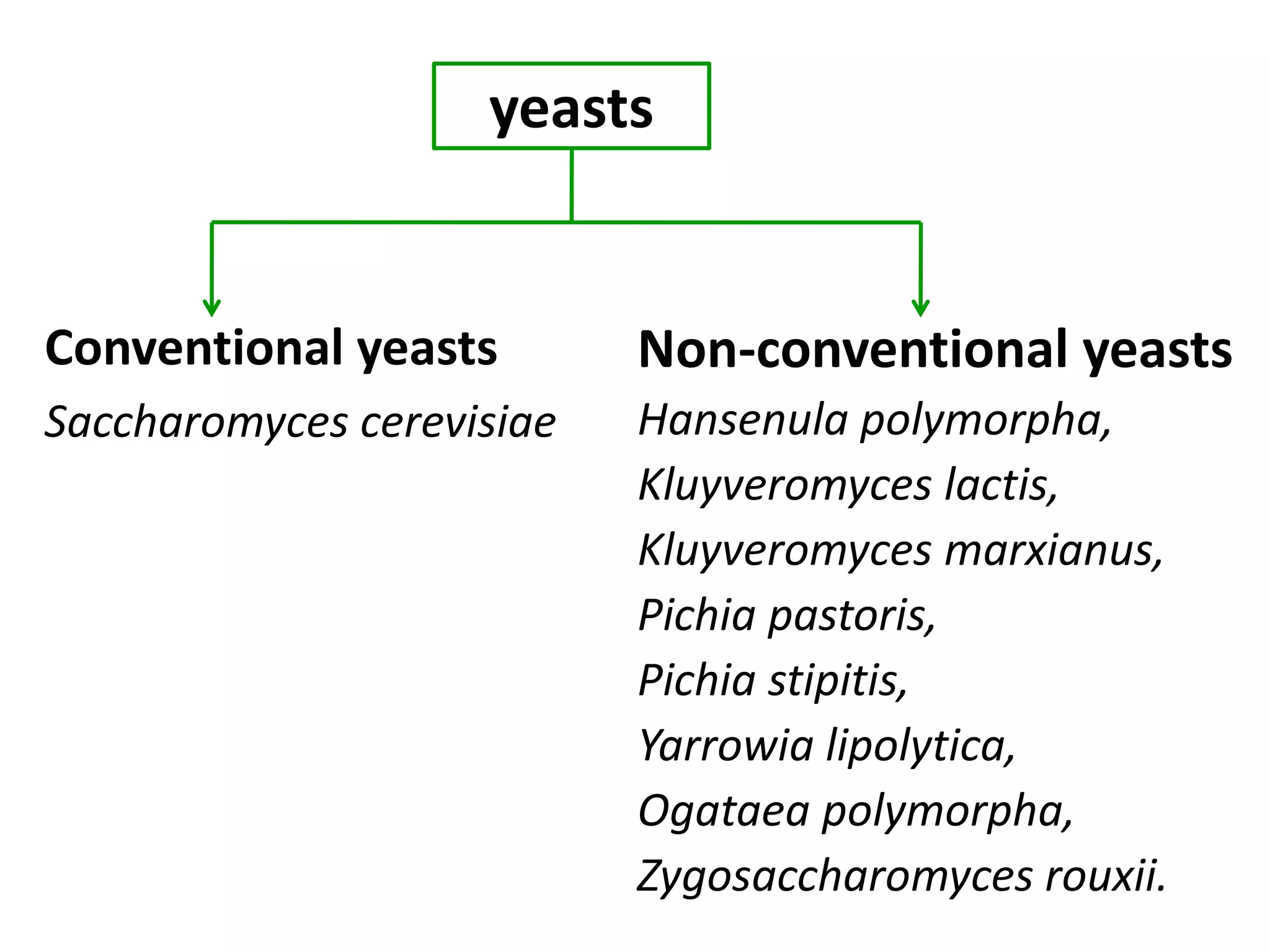
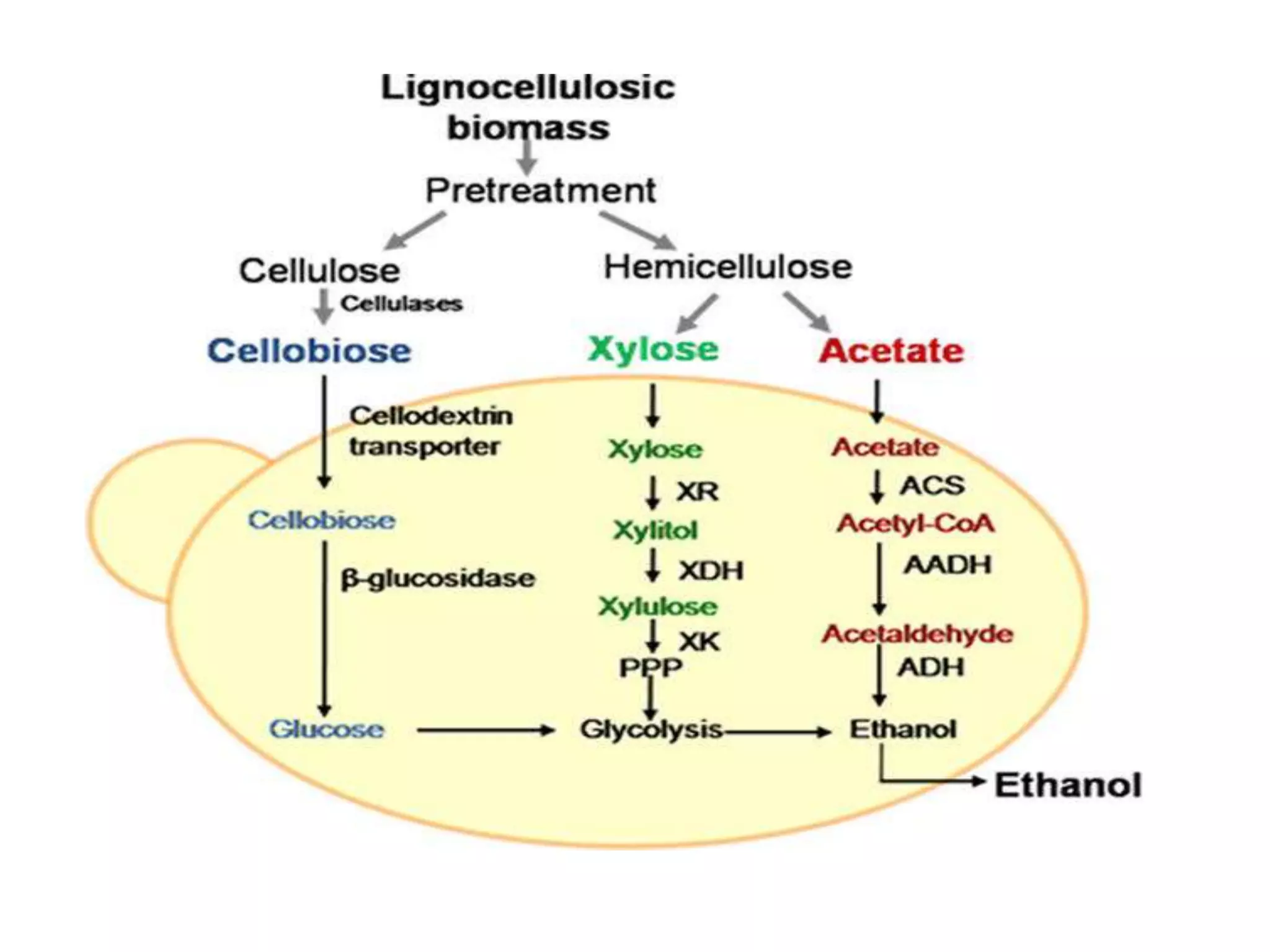
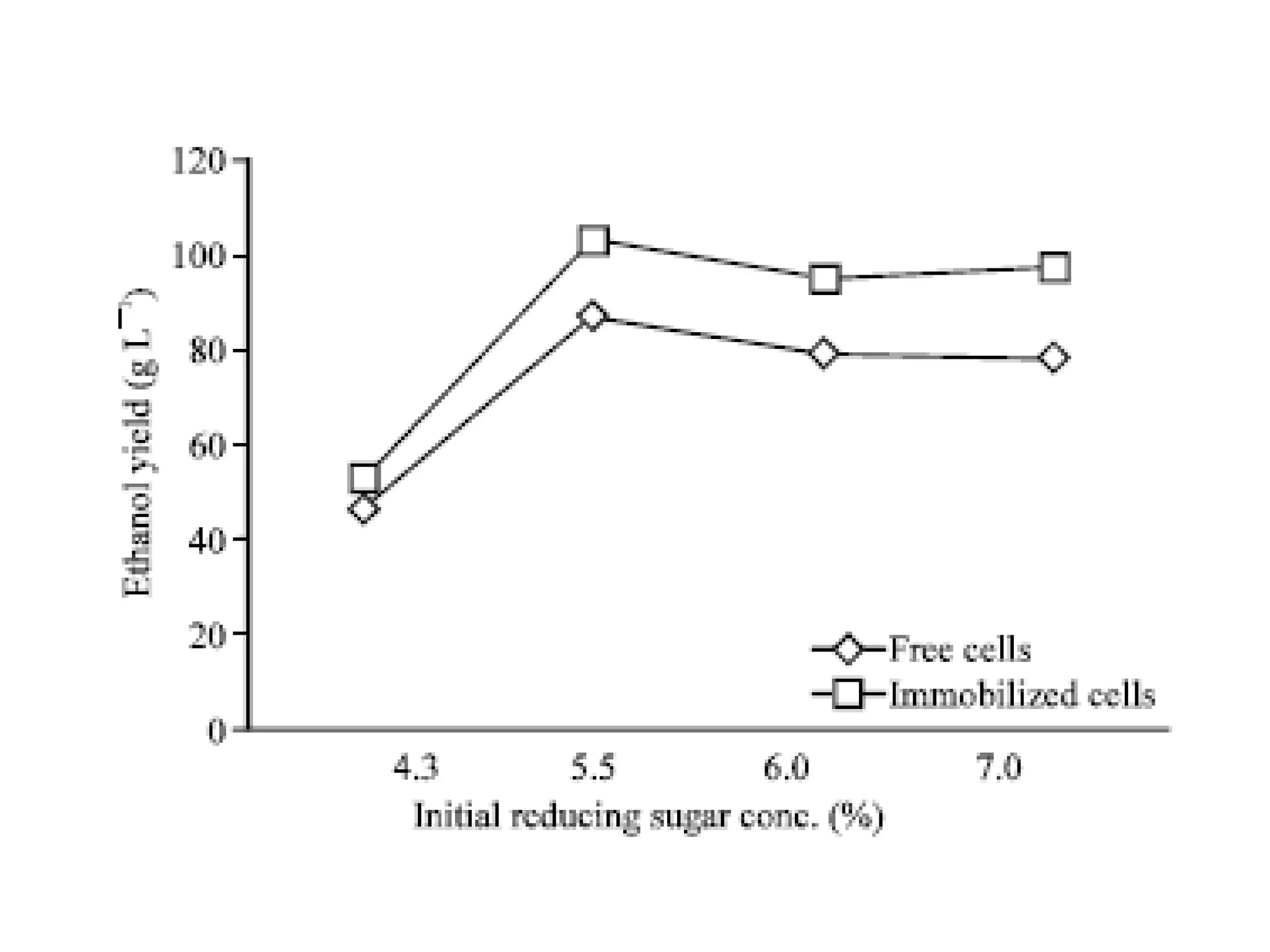
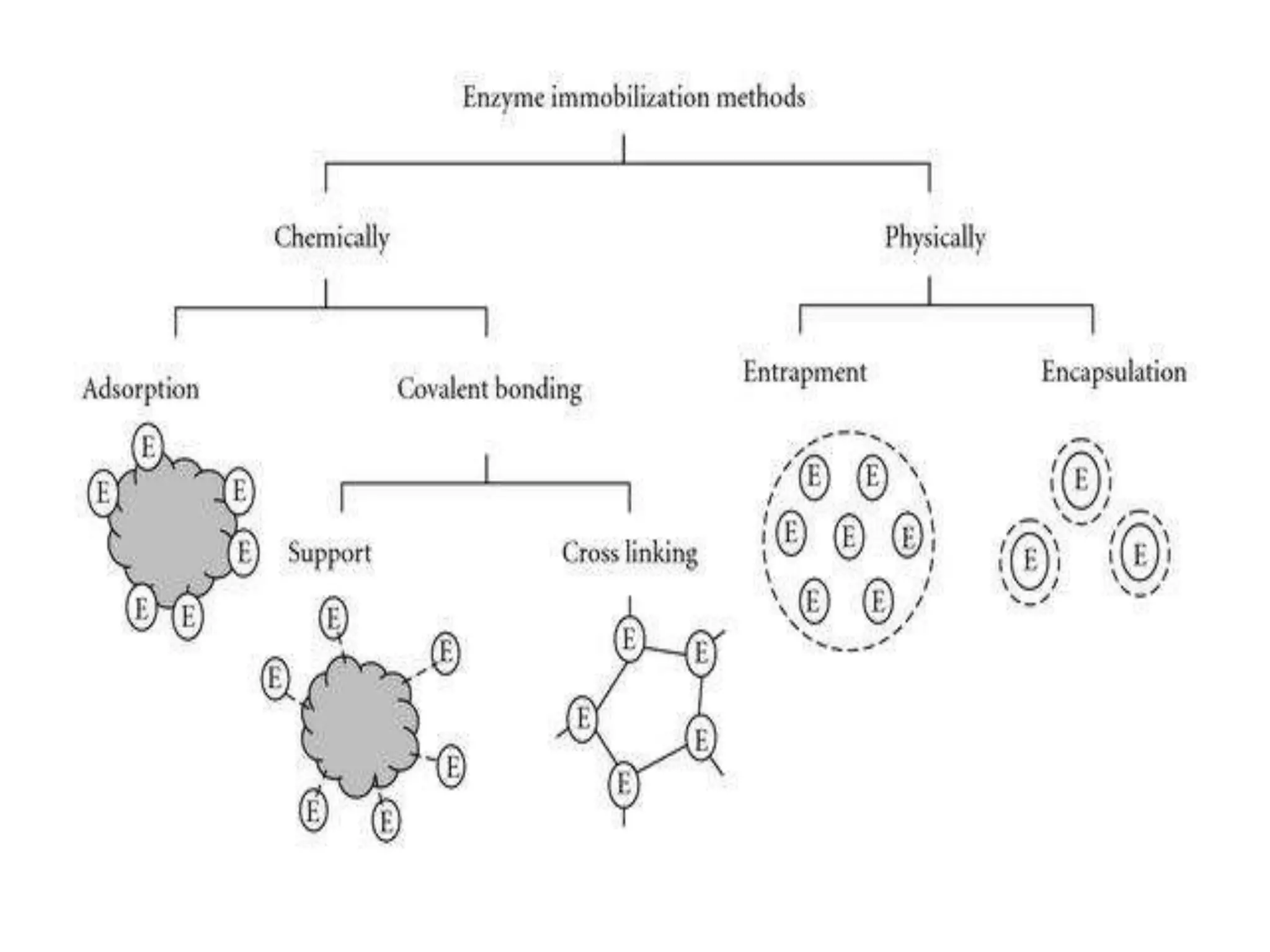
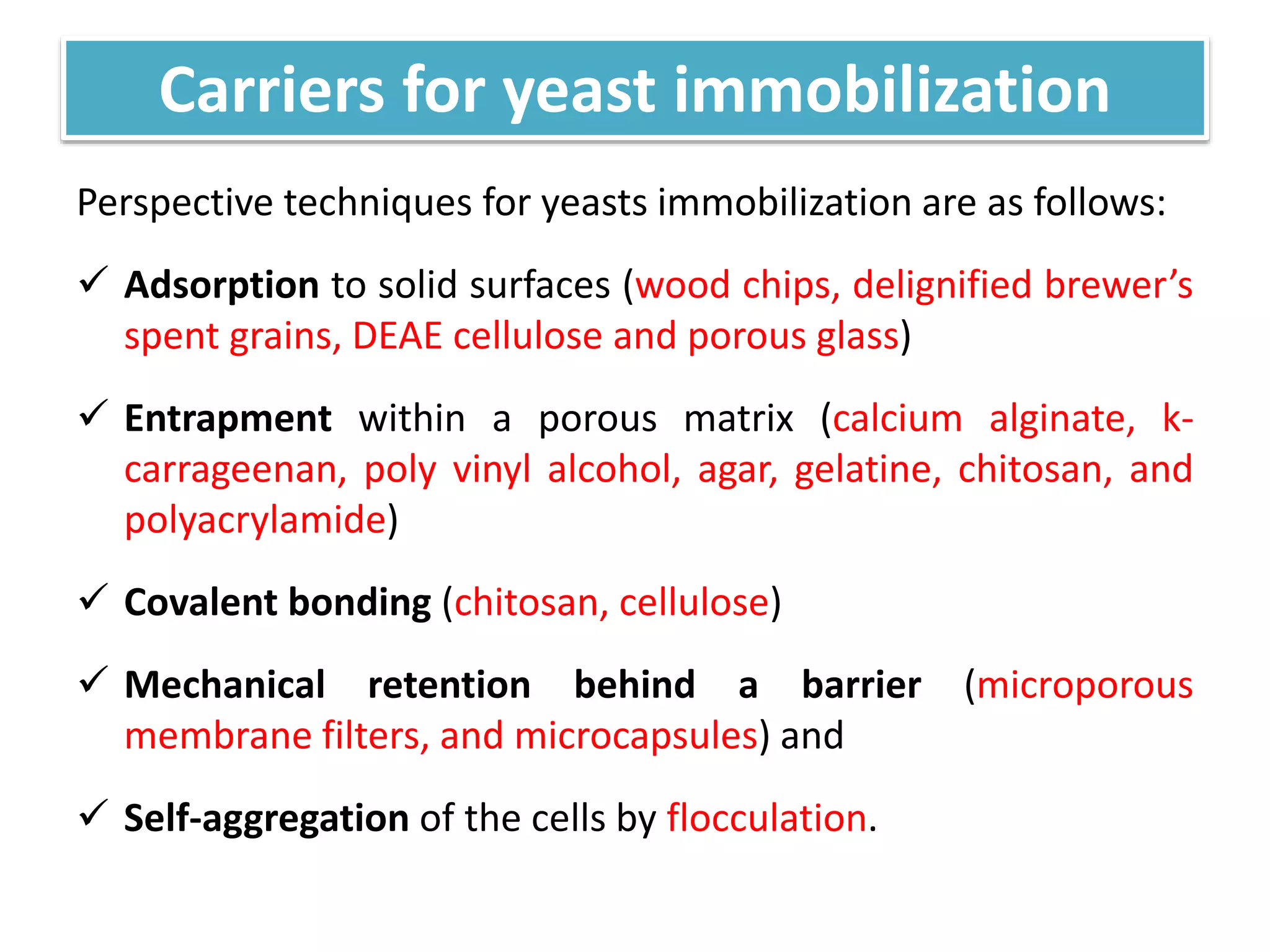
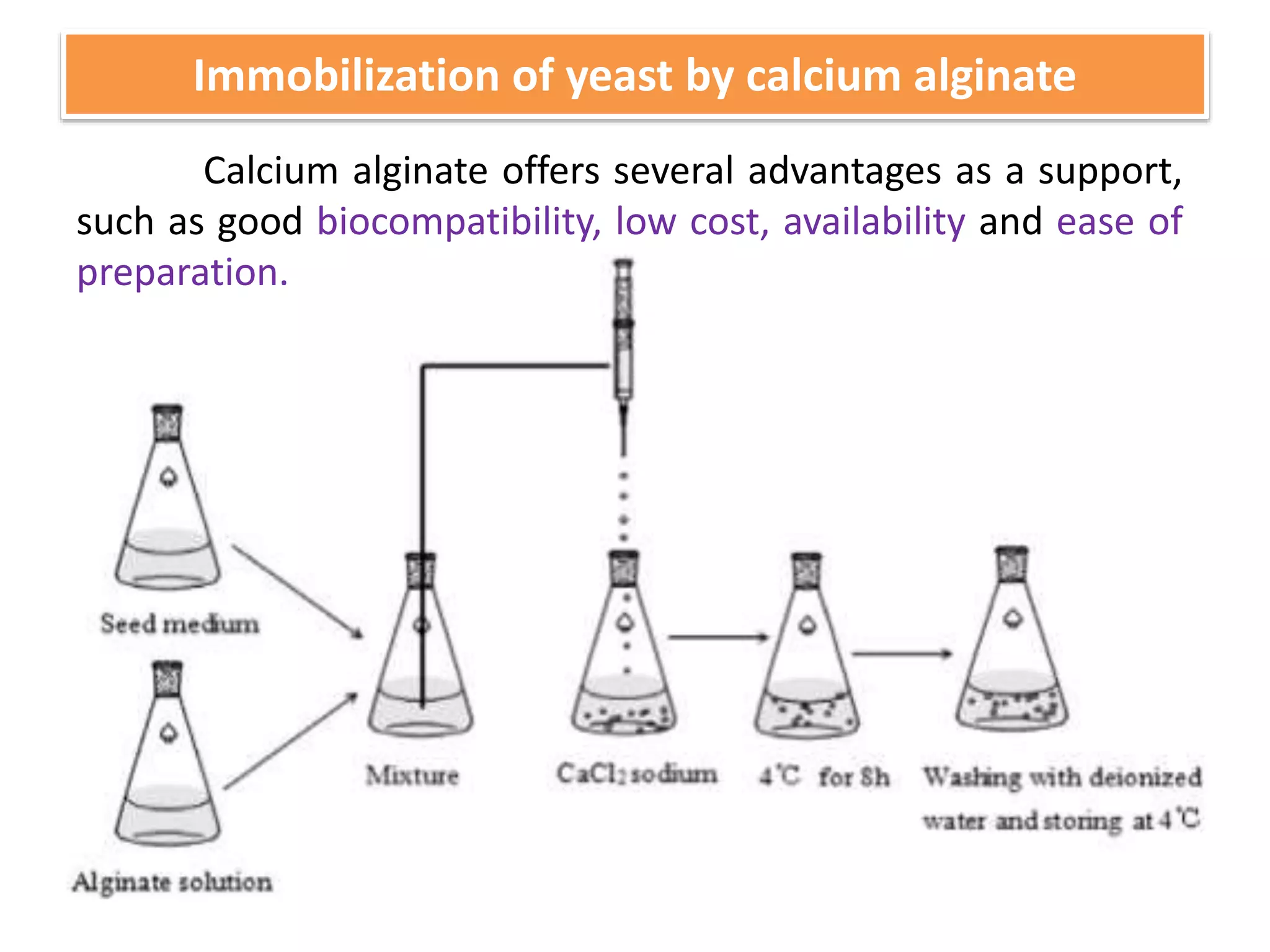
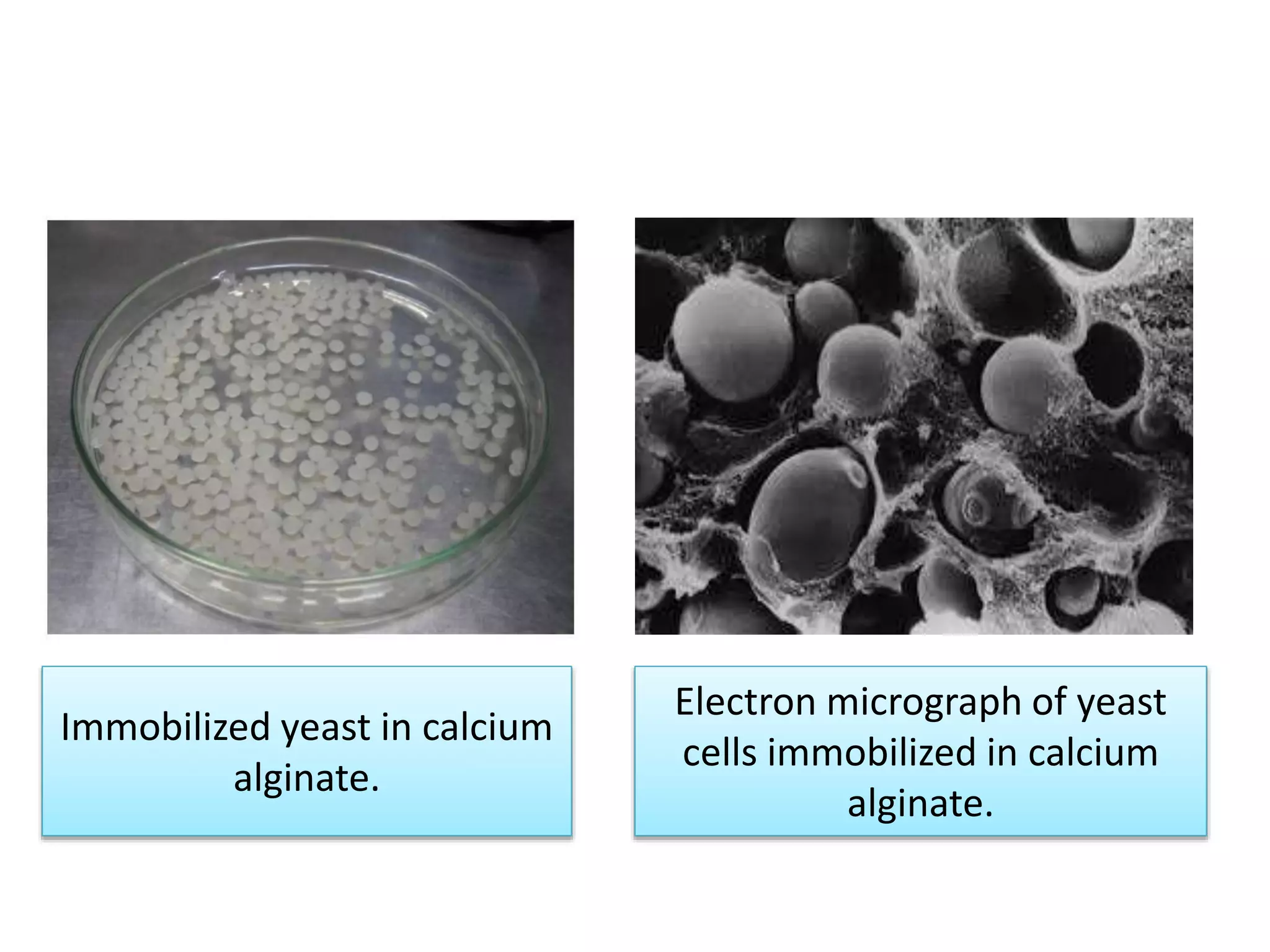
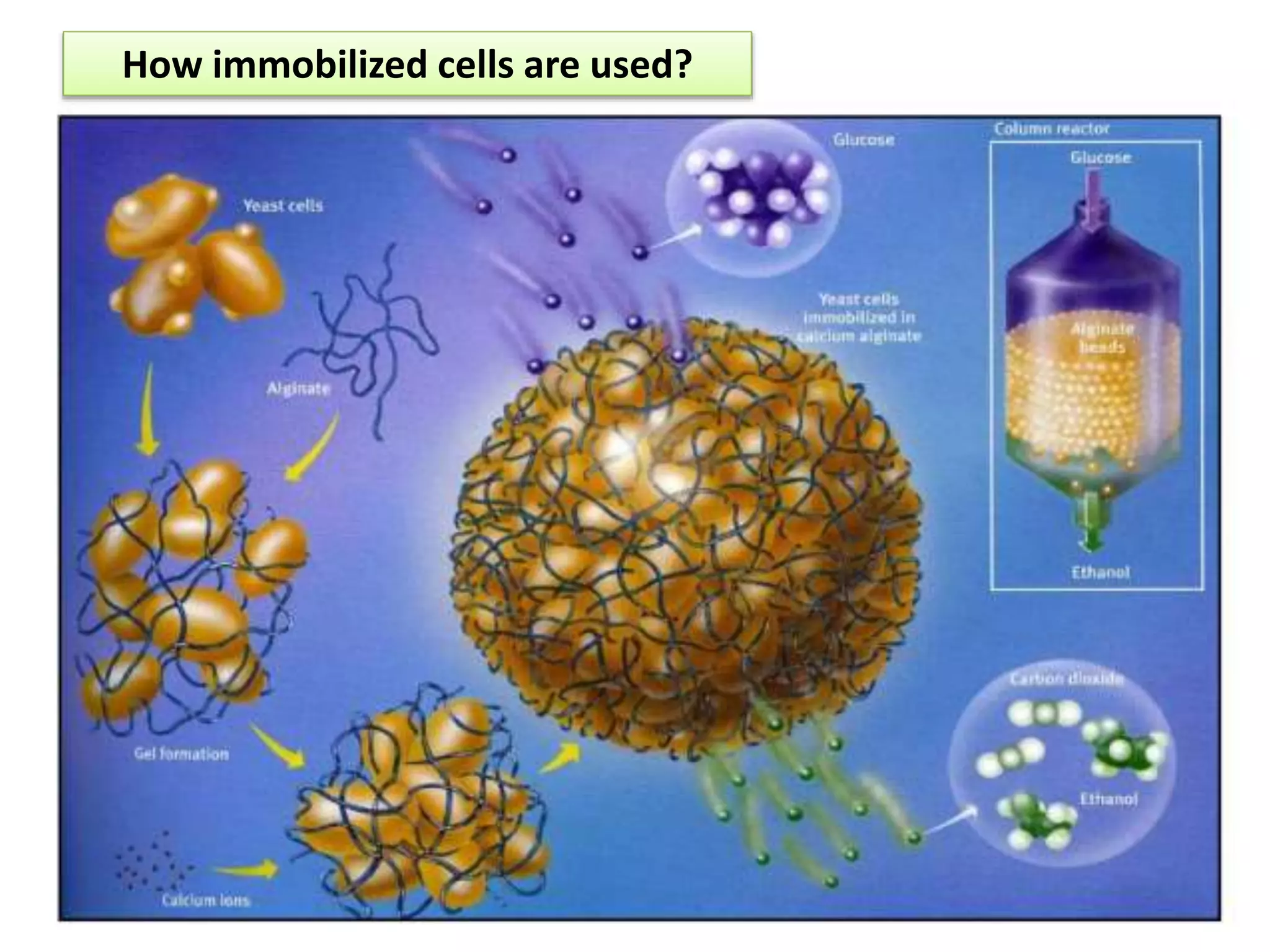
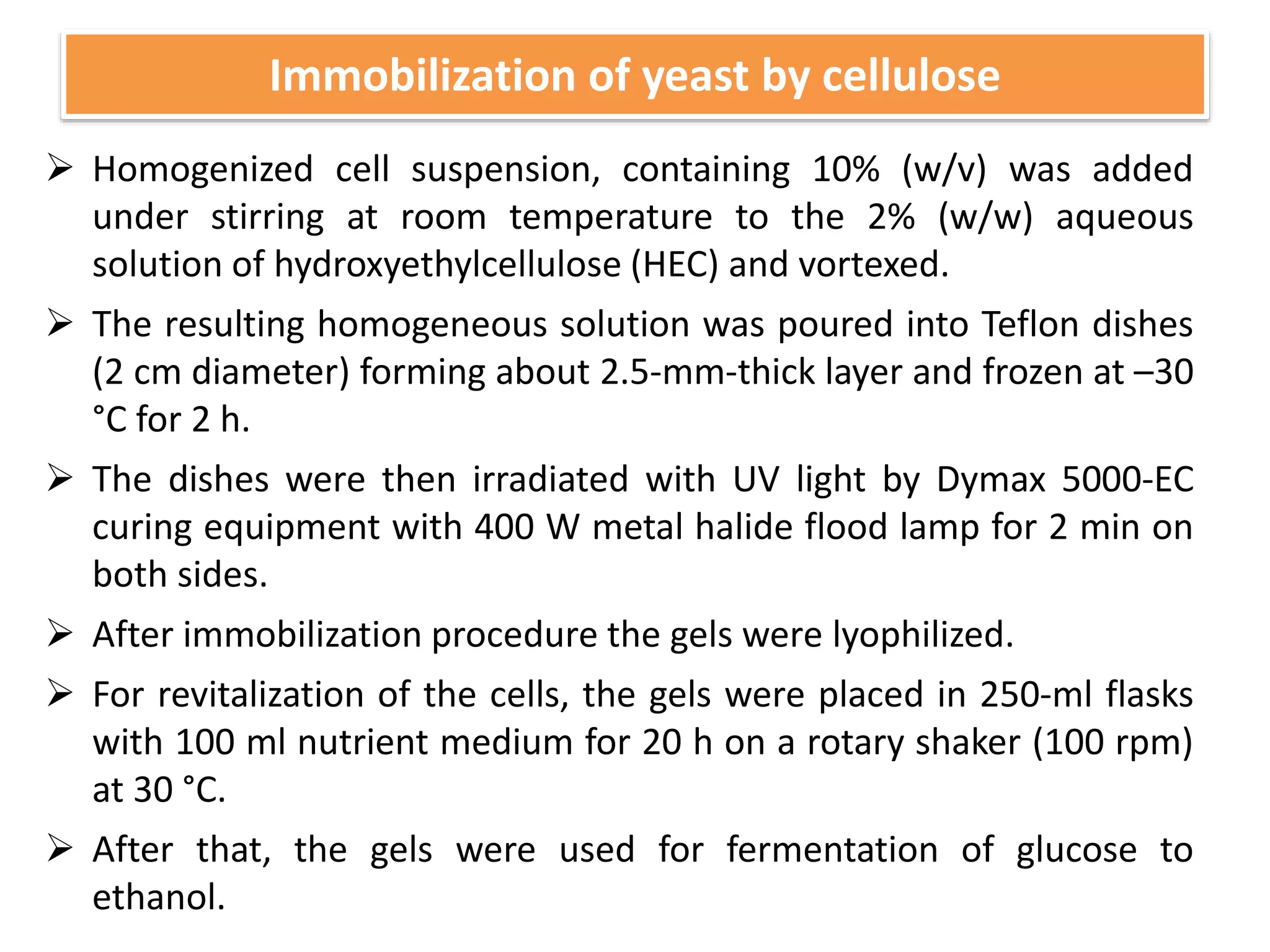
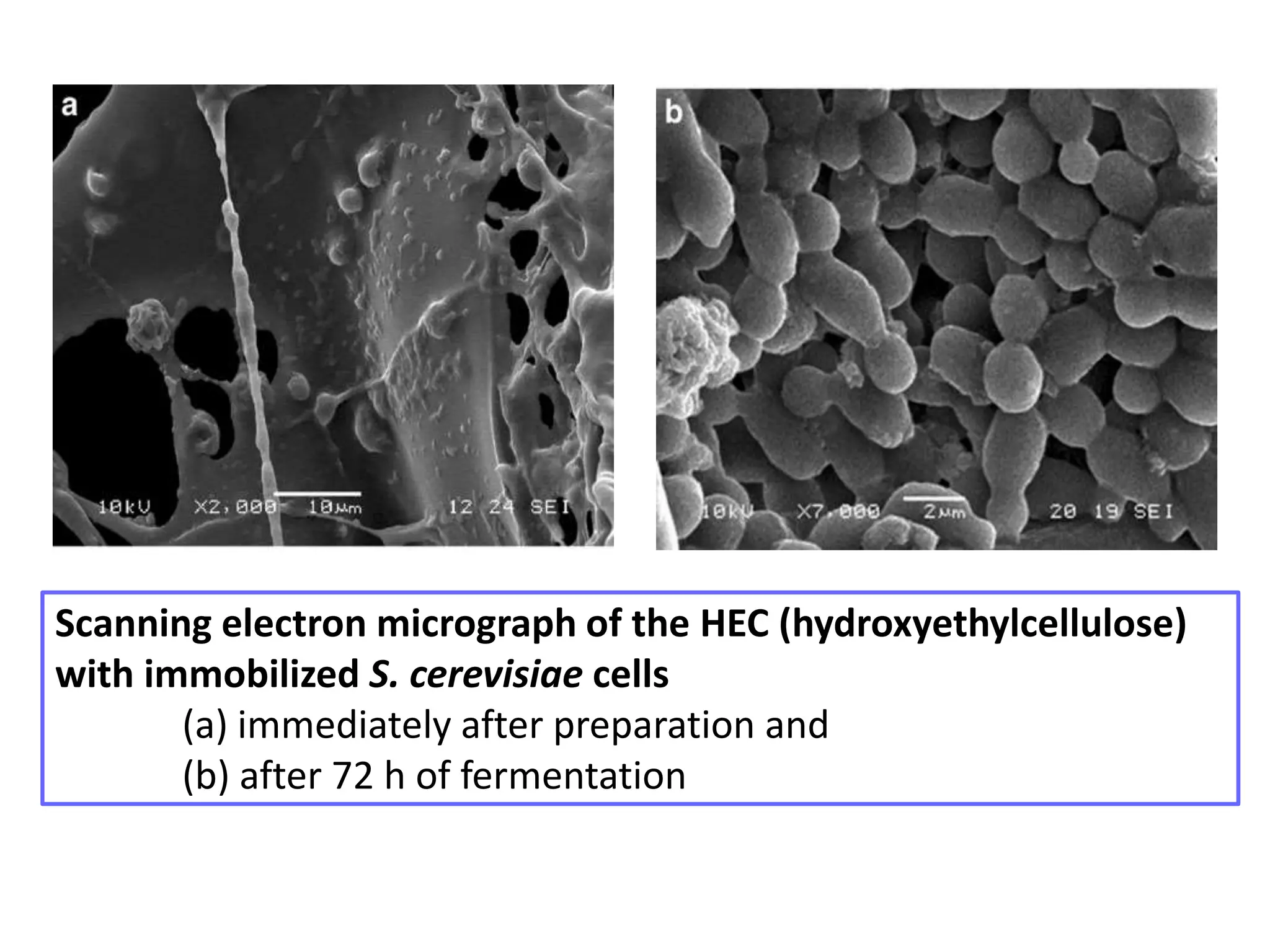
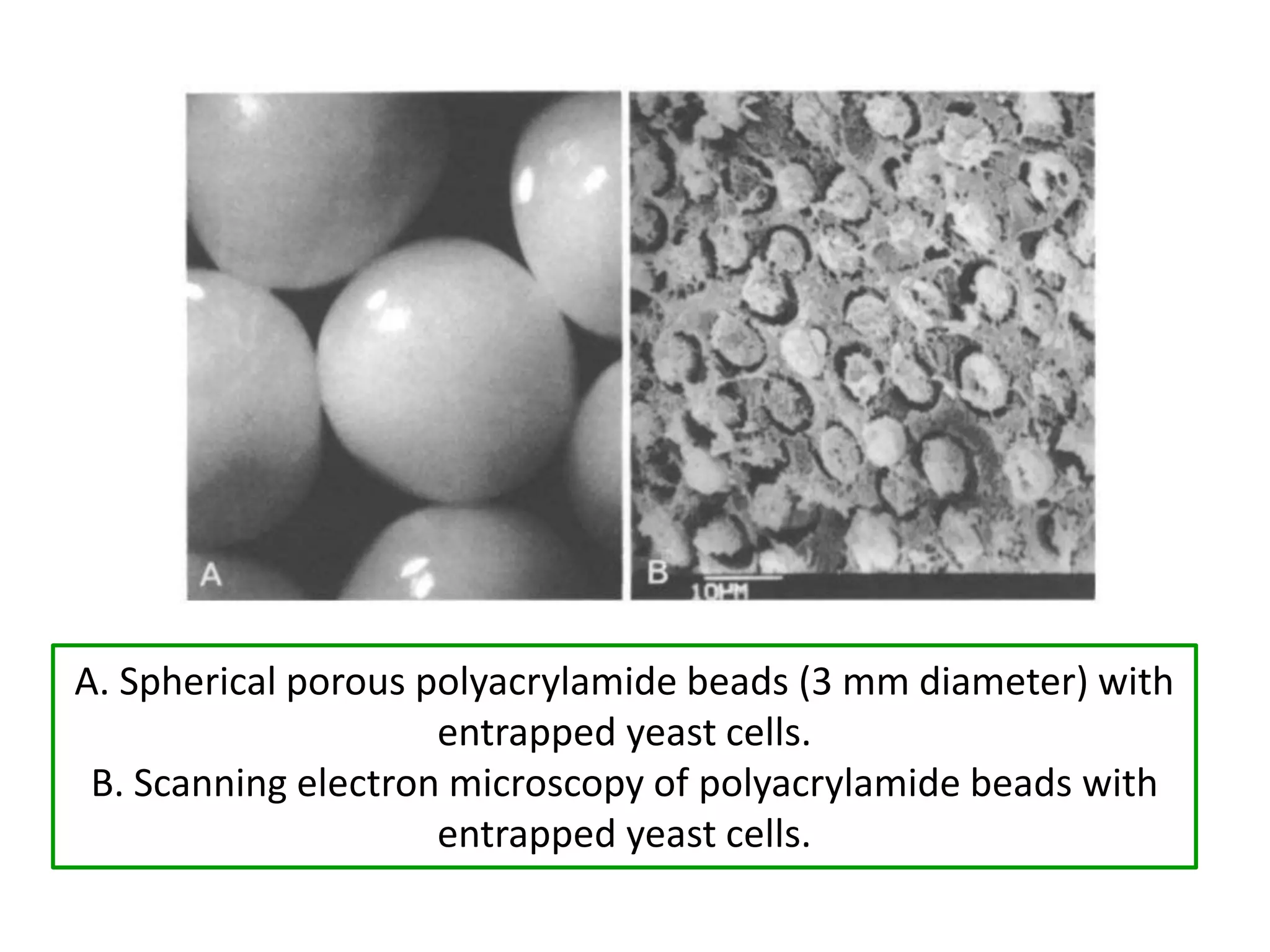
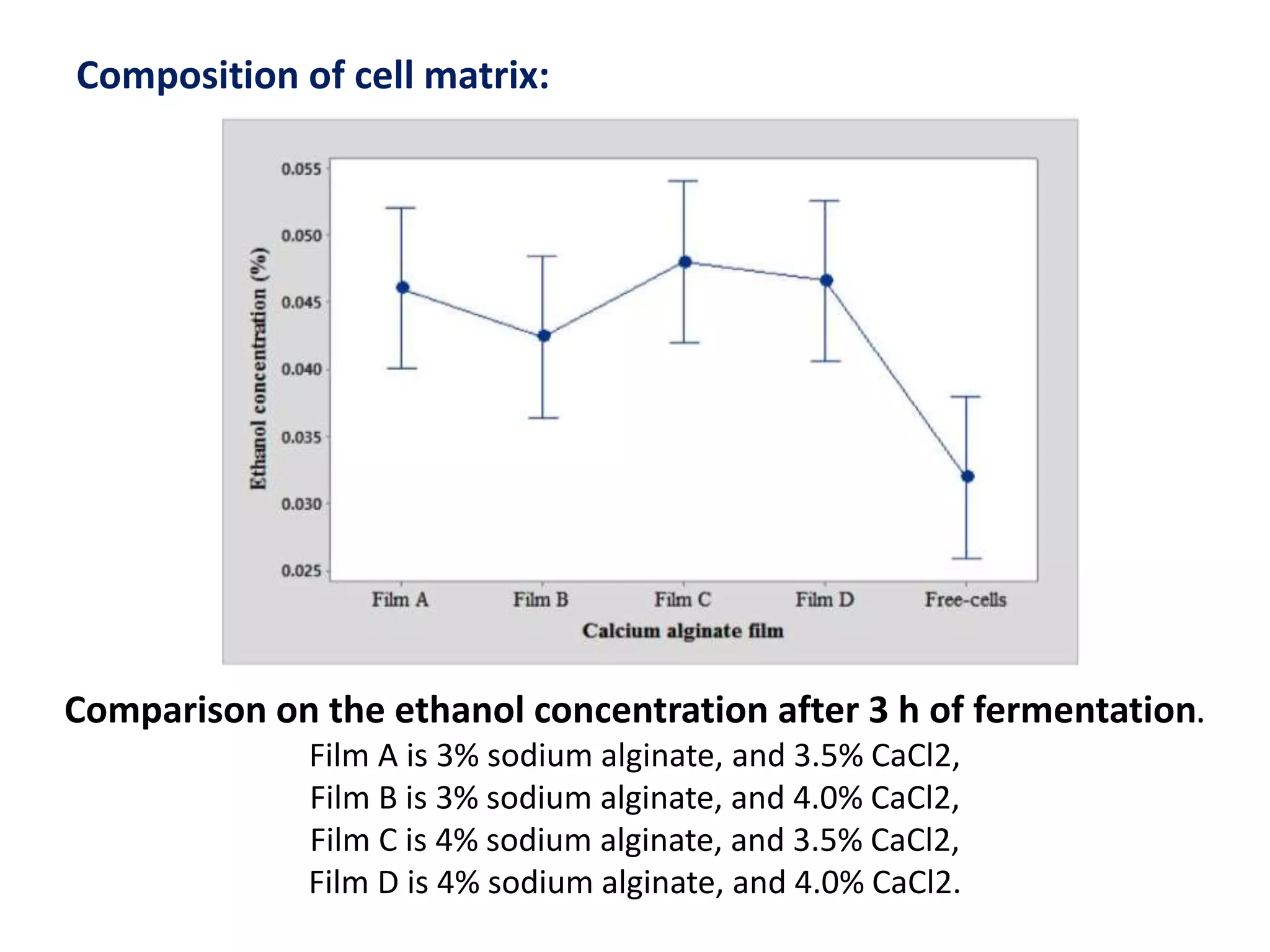
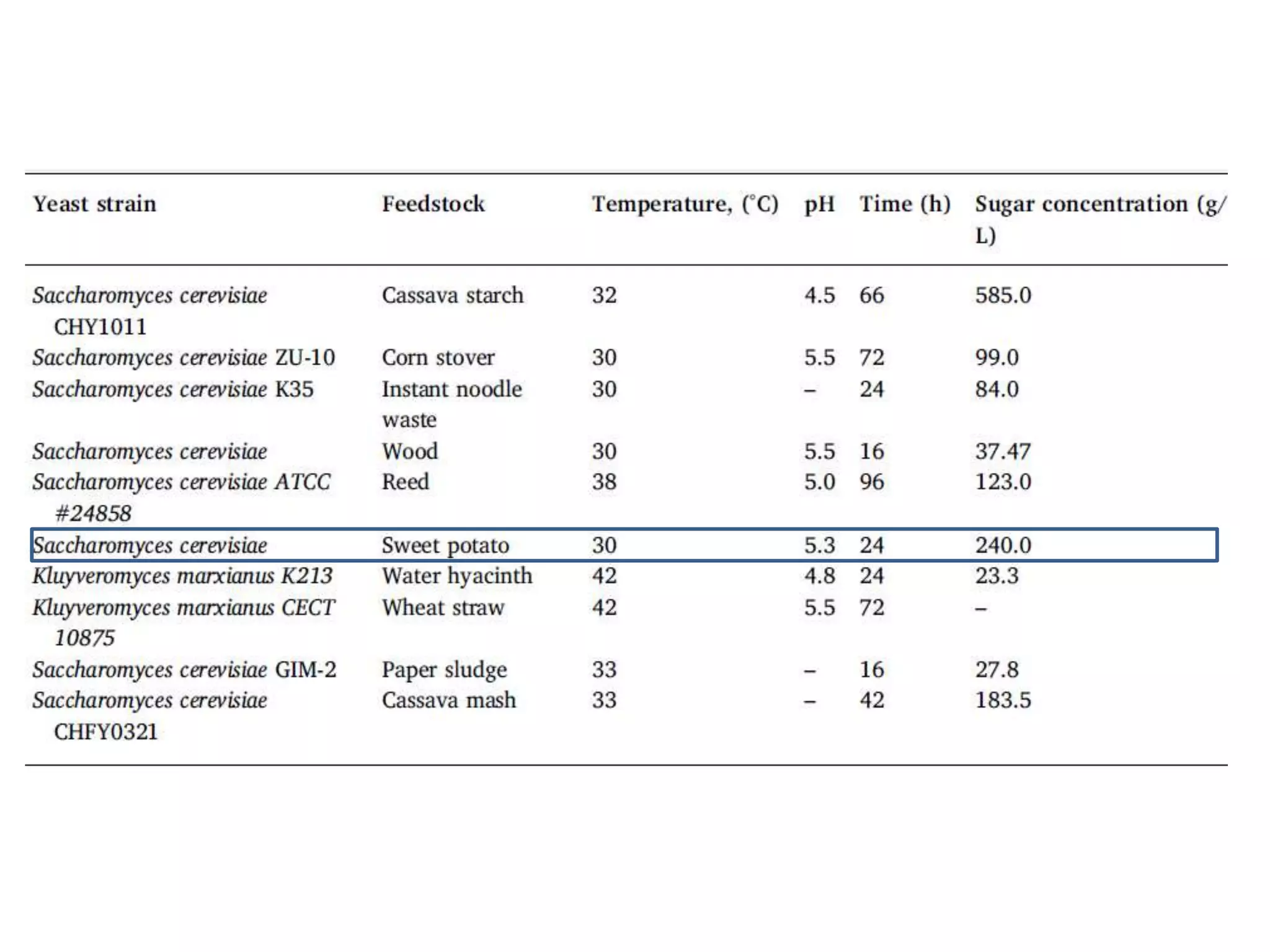
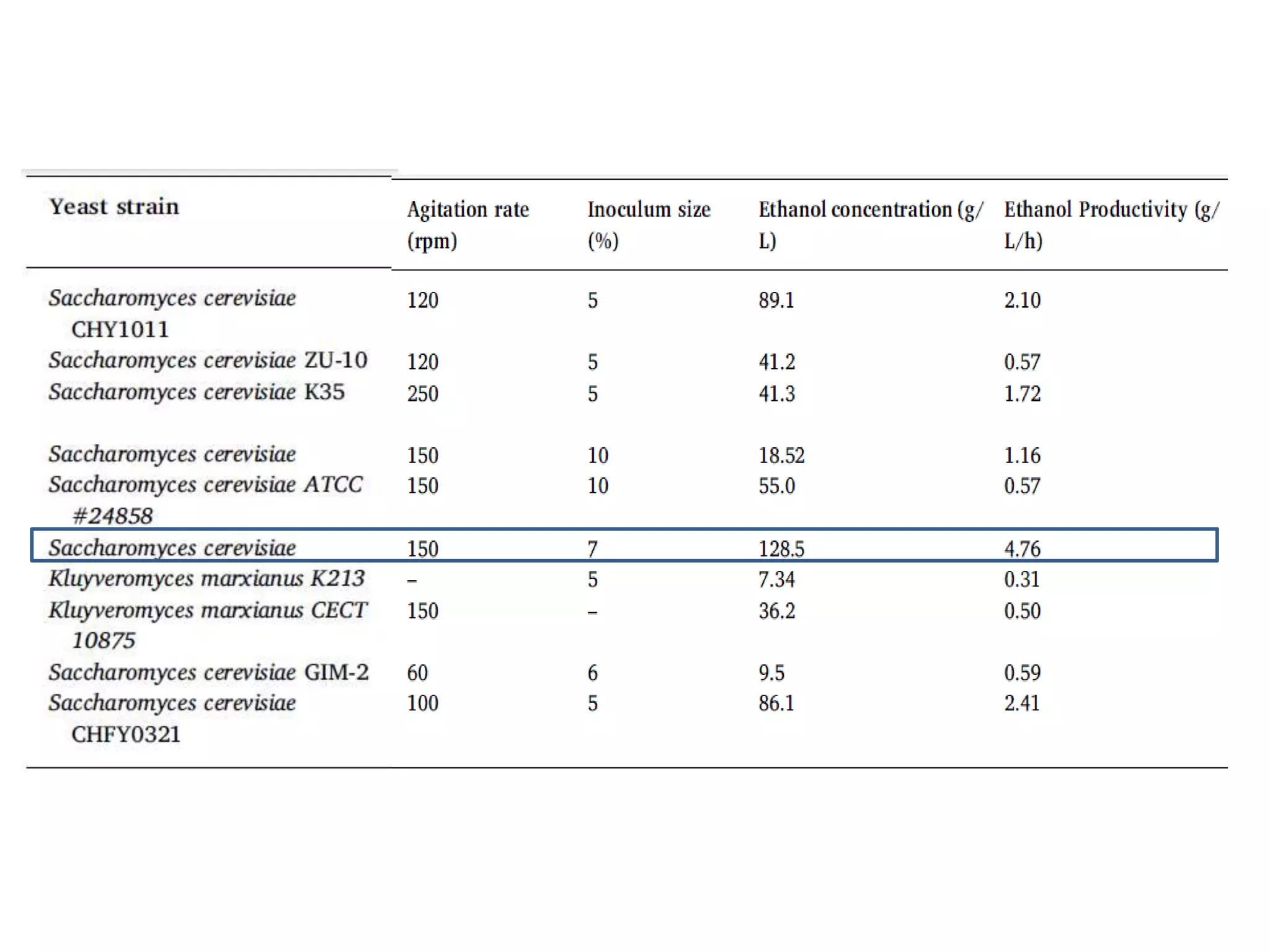
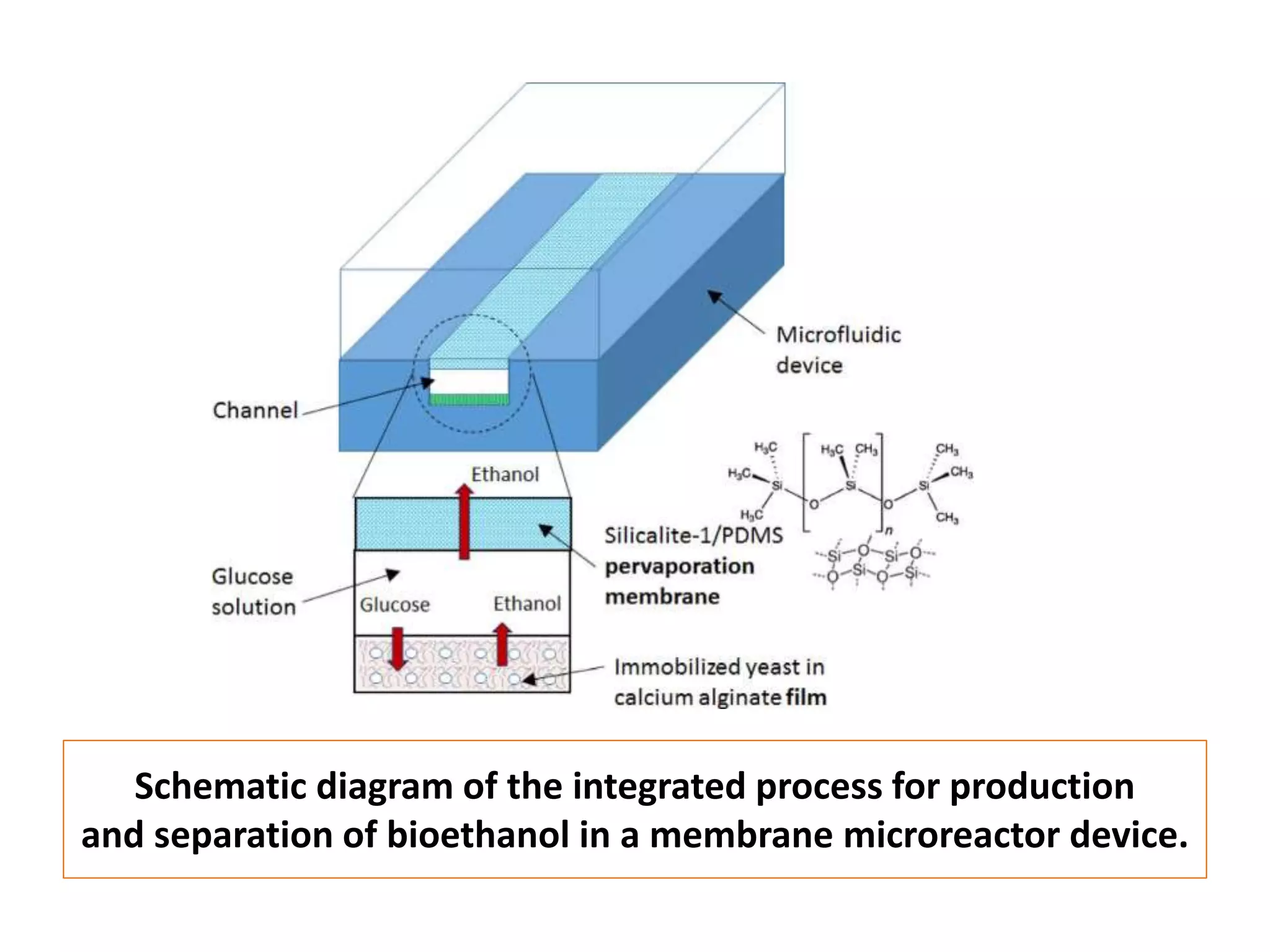
The document discusses various methods for immobilizing microbial cells, specifically yeast cells, for use in fermentation processes to produce bioethanol. It describes techniques like adsorption, entrapment in calcium alginate or polyacrylamide beads, and covalent bonding to supports like chitosan or cellulose. The immobilized yeast cells can be used for continuous fermentation with controls over factors like temperature, sugar concentration, pH, agitation rate and inoculum size to optimize ethanol production. Immobilization provides benefits like reuse of cells and easier product separation using techniques like membrane microreactors.