The document provides an outline and notes on the topics of rate of reaction, collision theory, factors affecting reaction rates, activation energy, endothermic and exothermic reactions, equilibrium, and Le Chatelier's principle. Key points include:
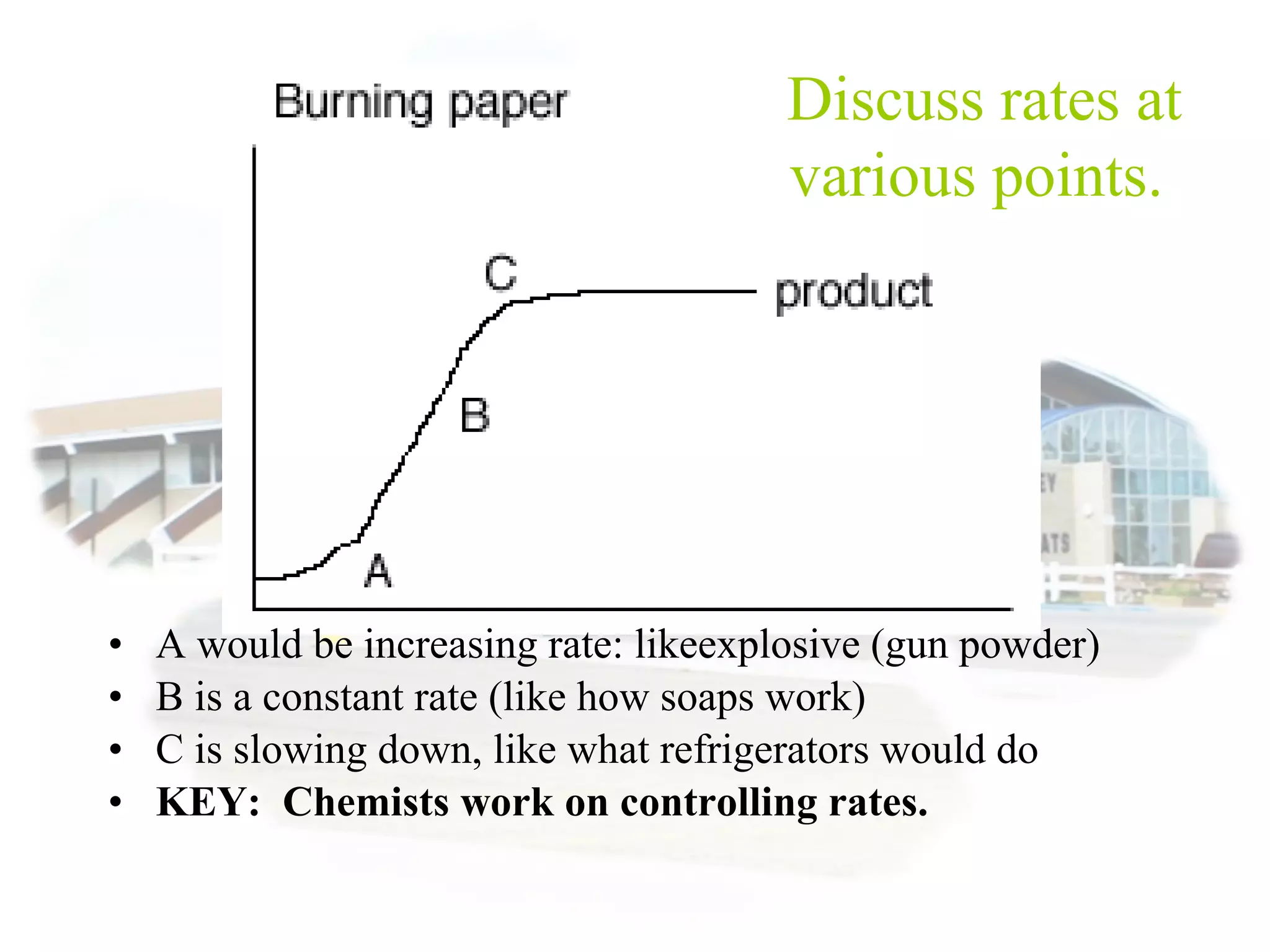
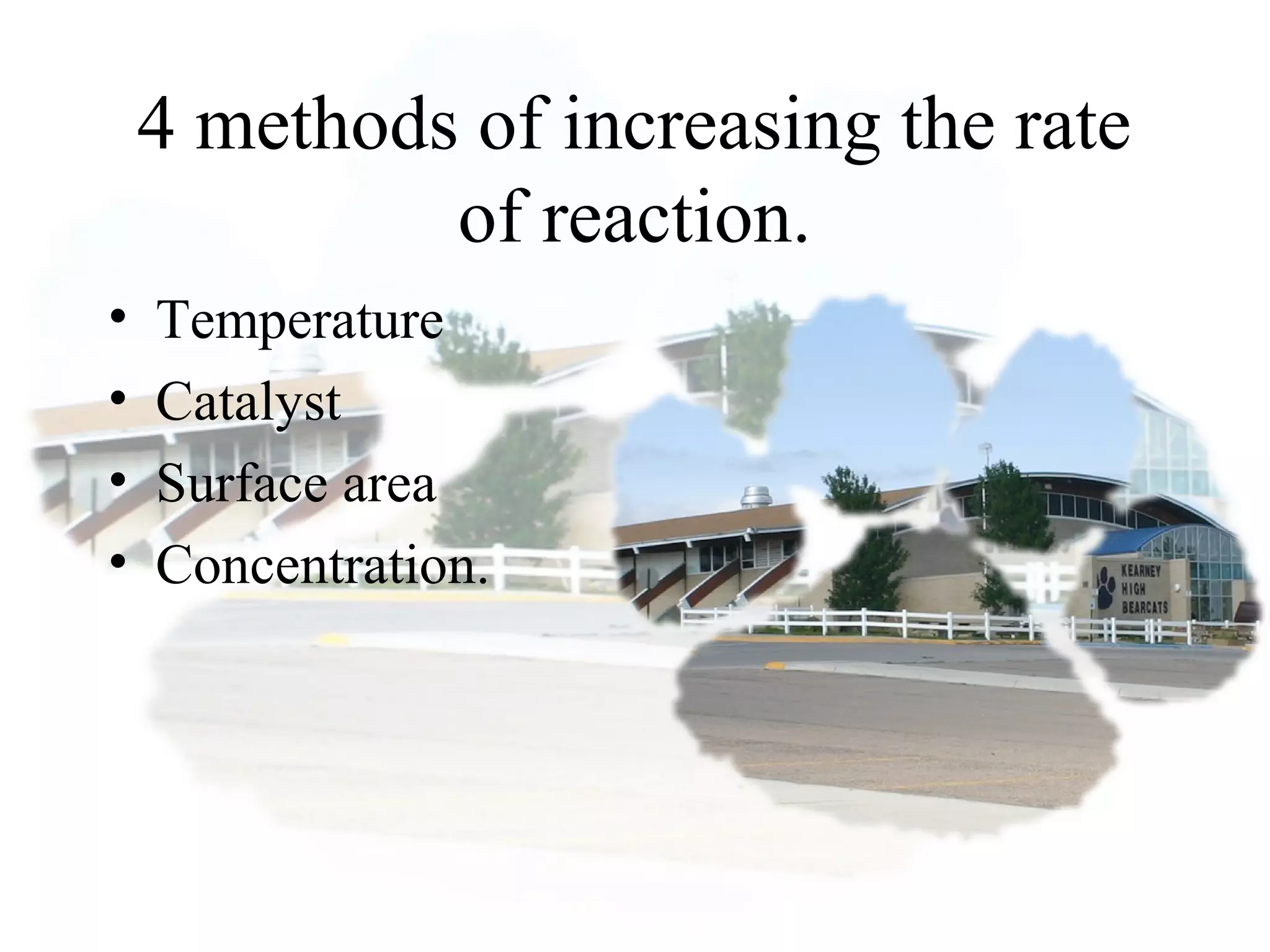
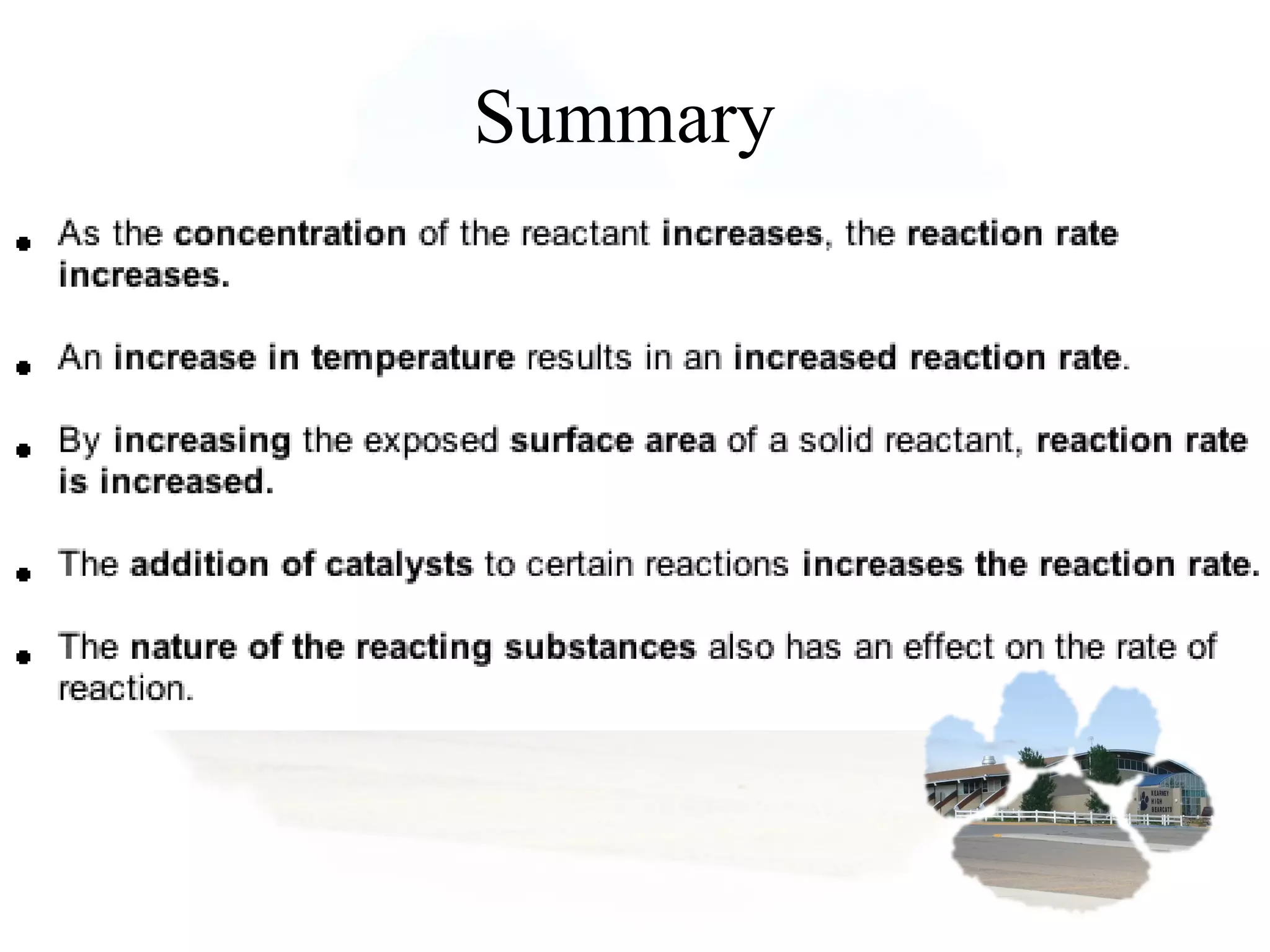
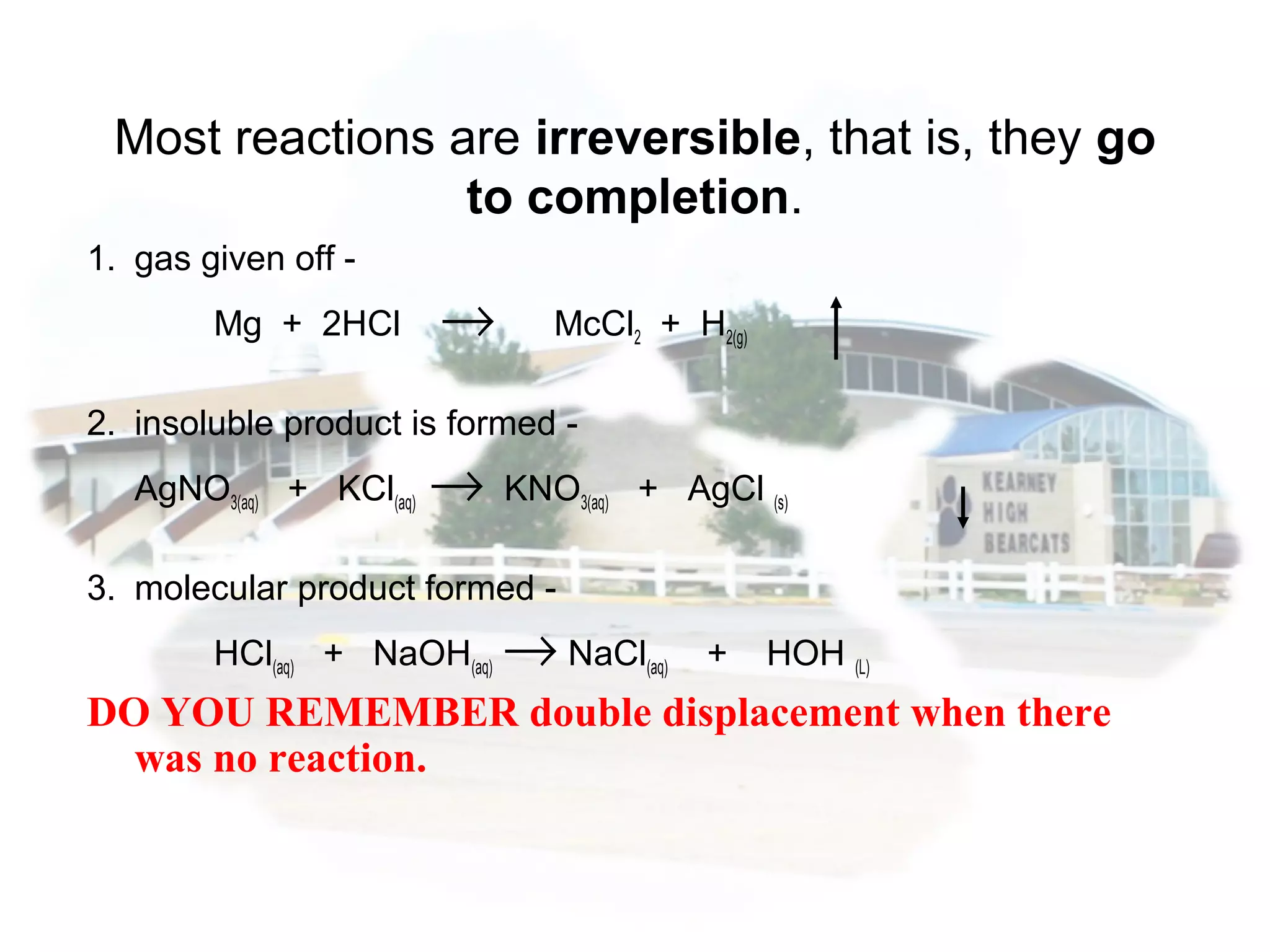
- Reaction rates can be determined by measuring how quickly reactants are used up or products are formed. Temperature, catalysts, surface area, and concentration can impact reaction rates.
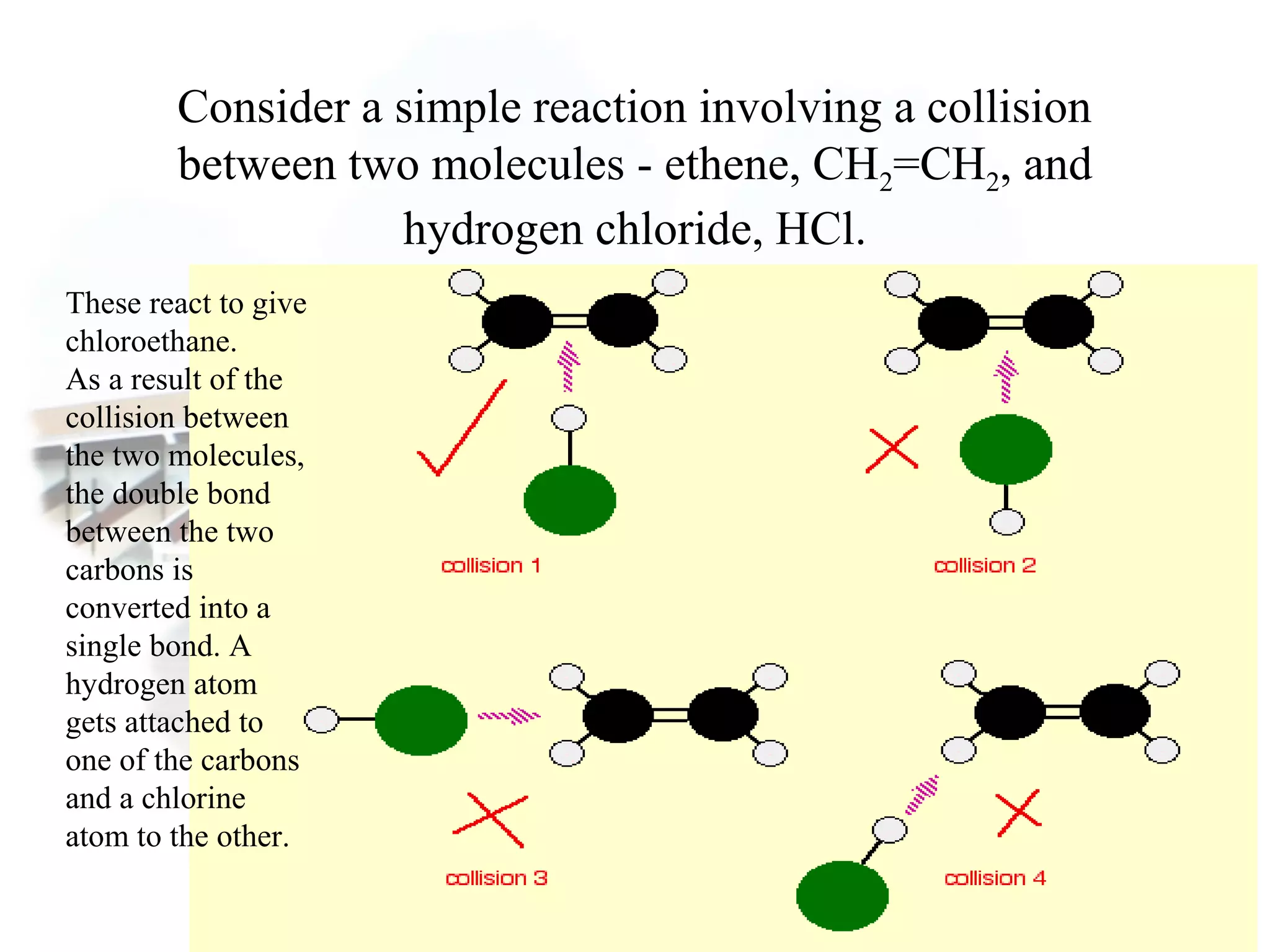
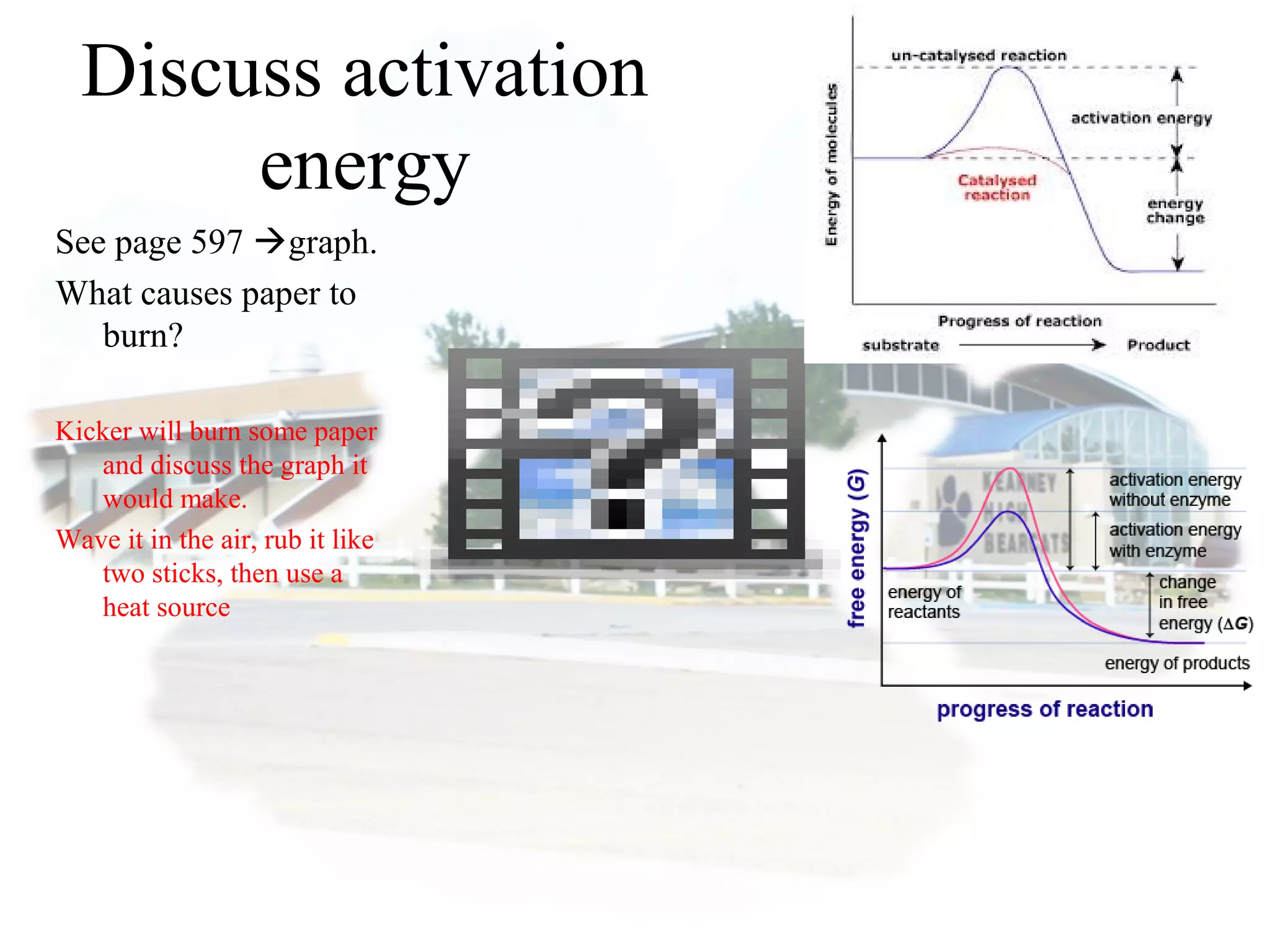
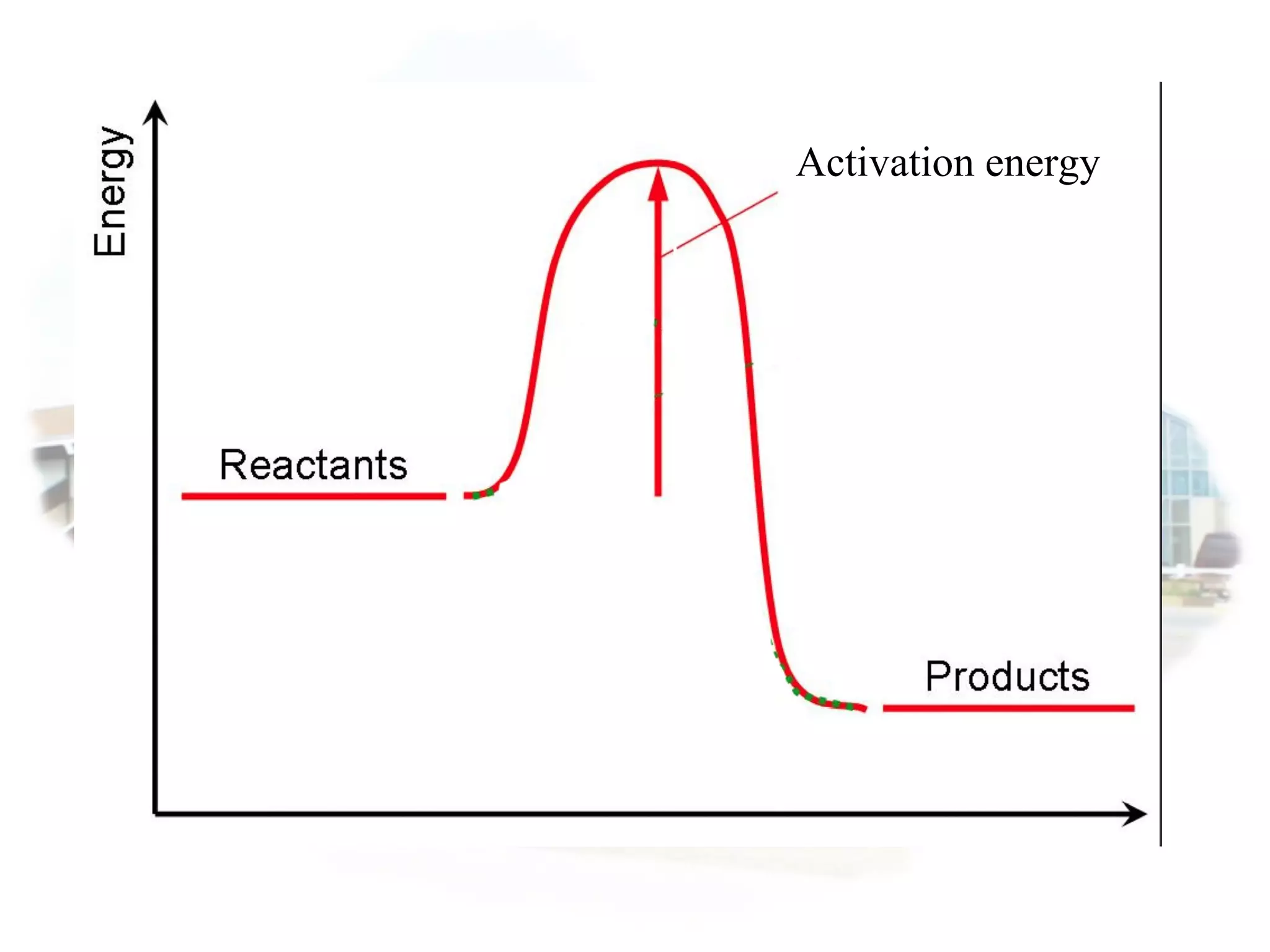
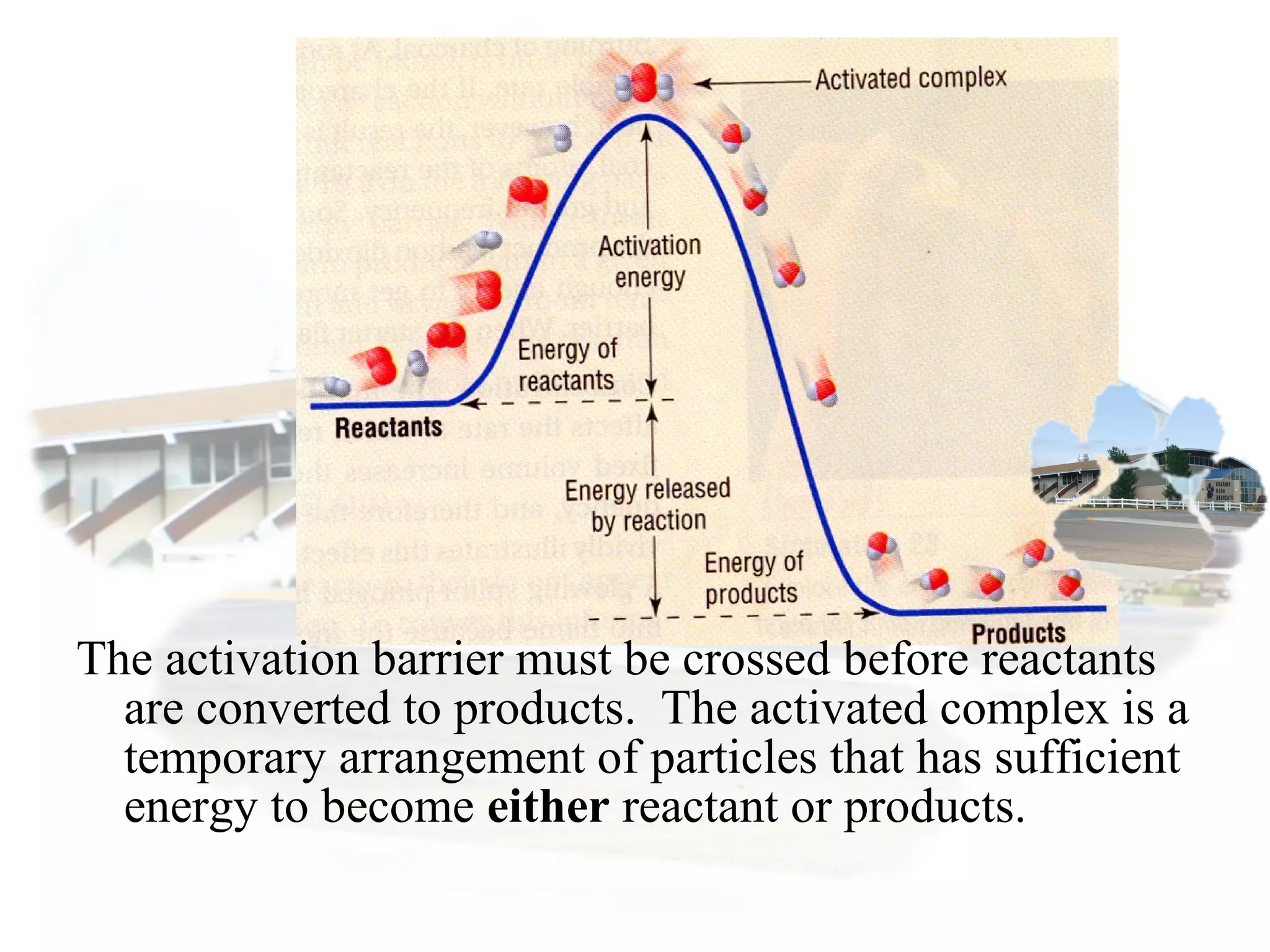
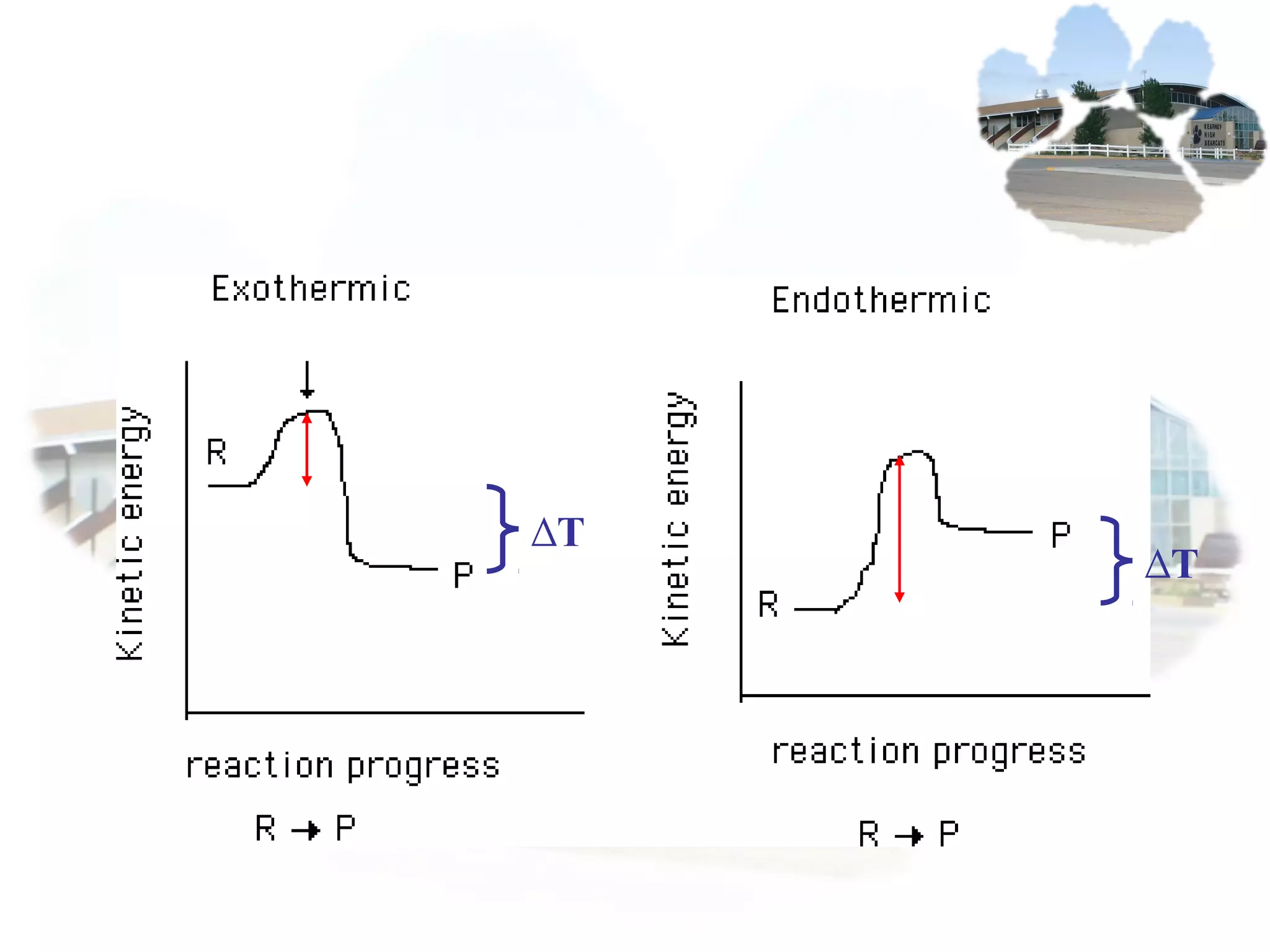
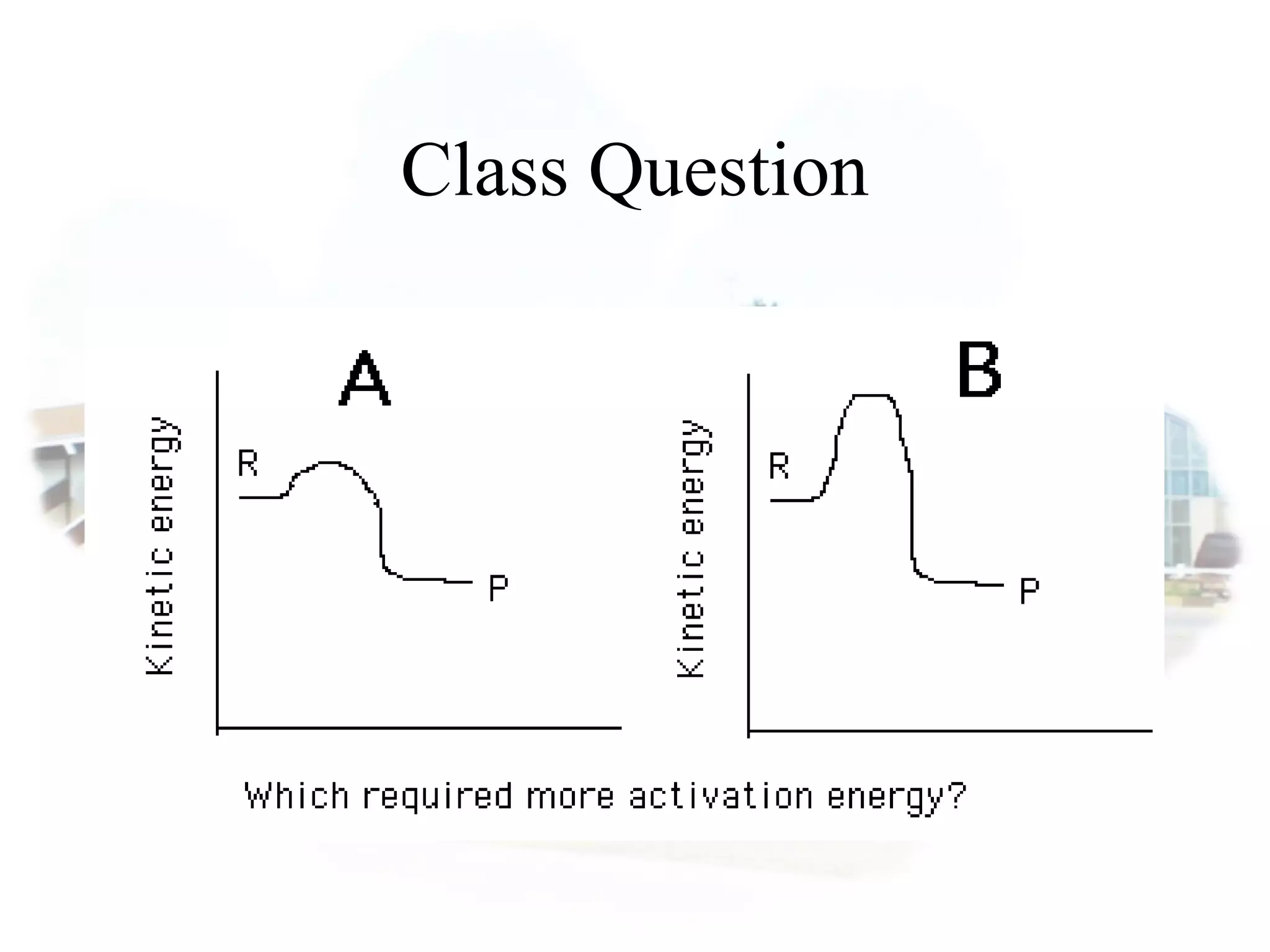
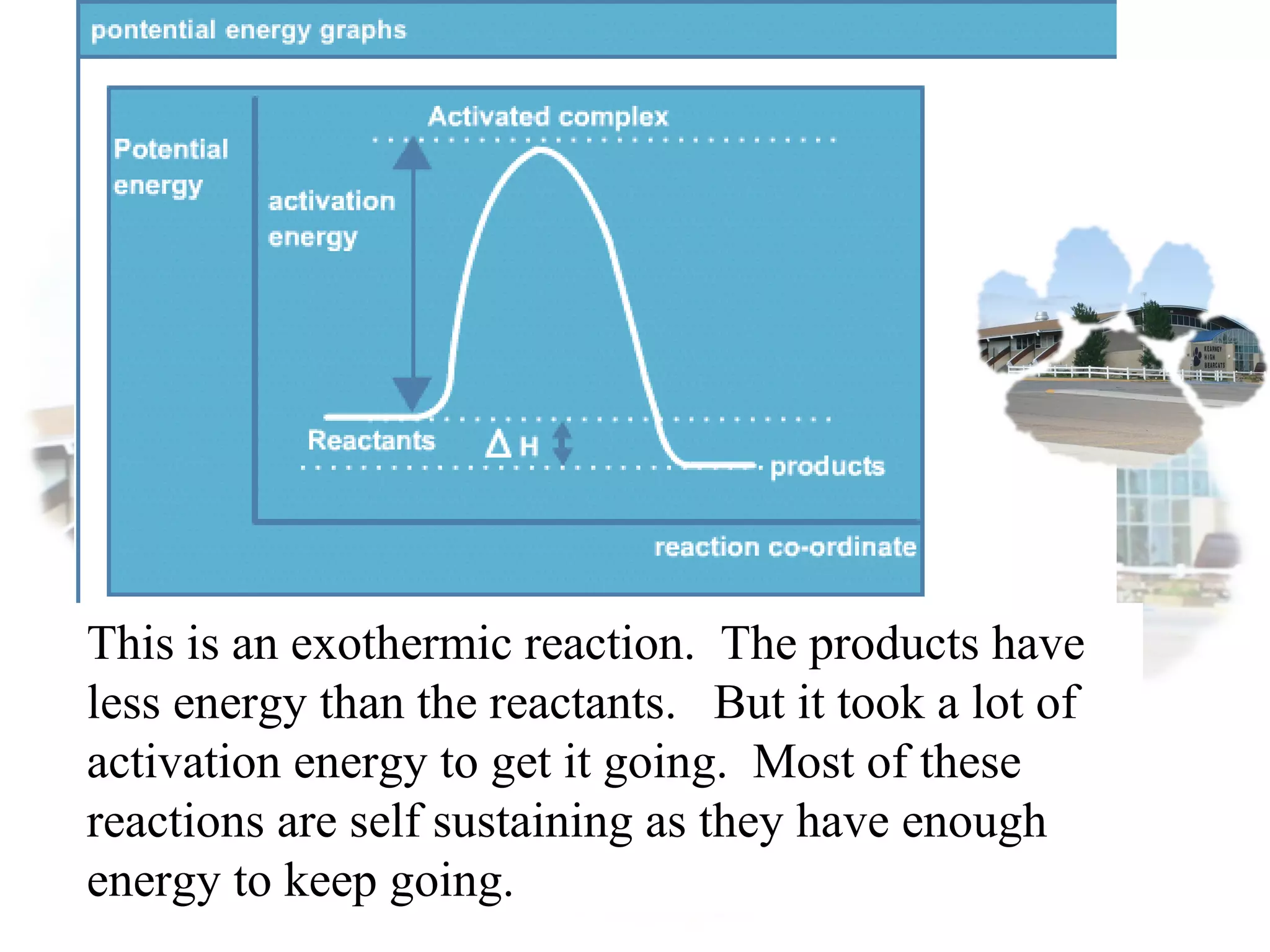
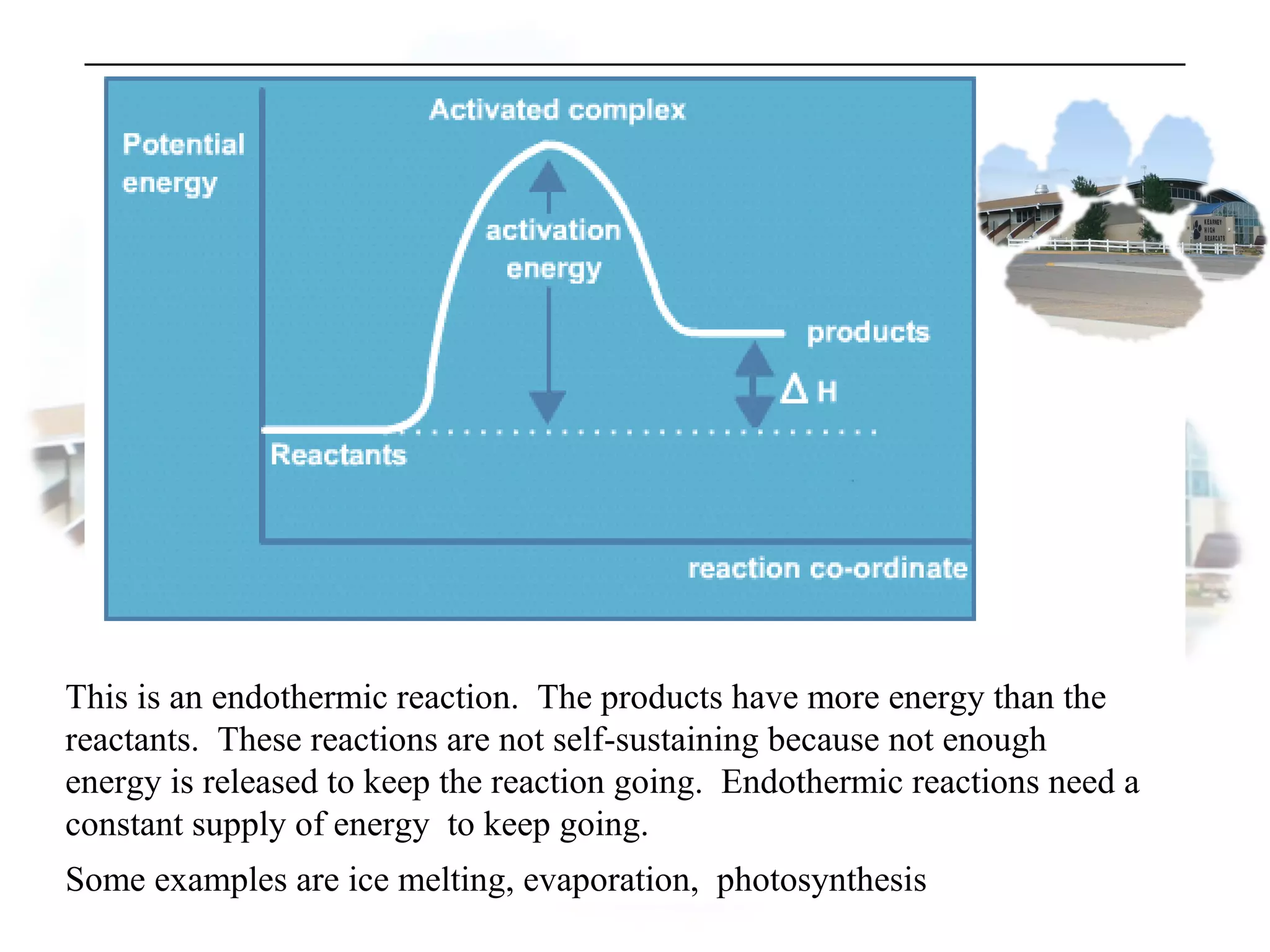
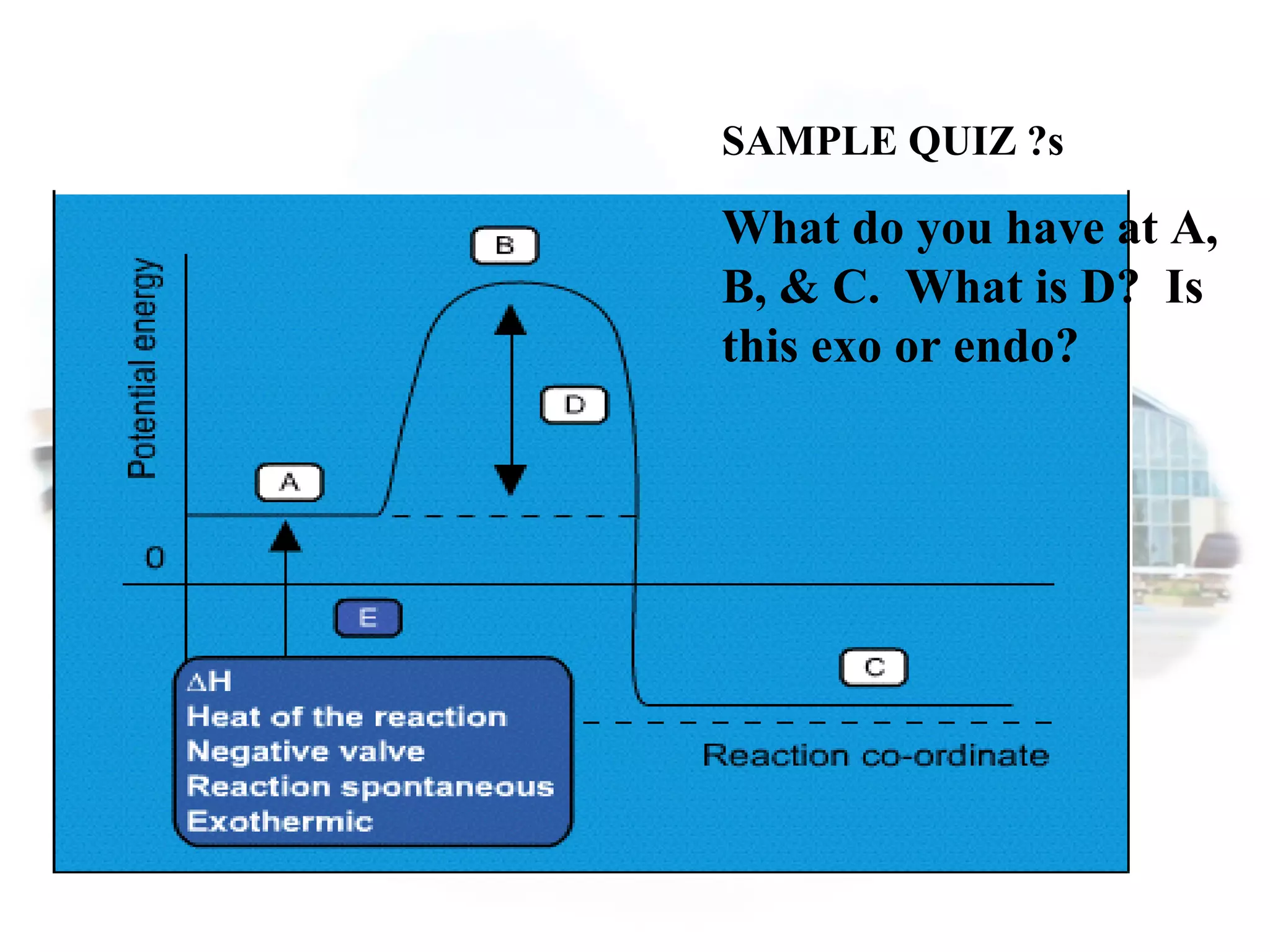
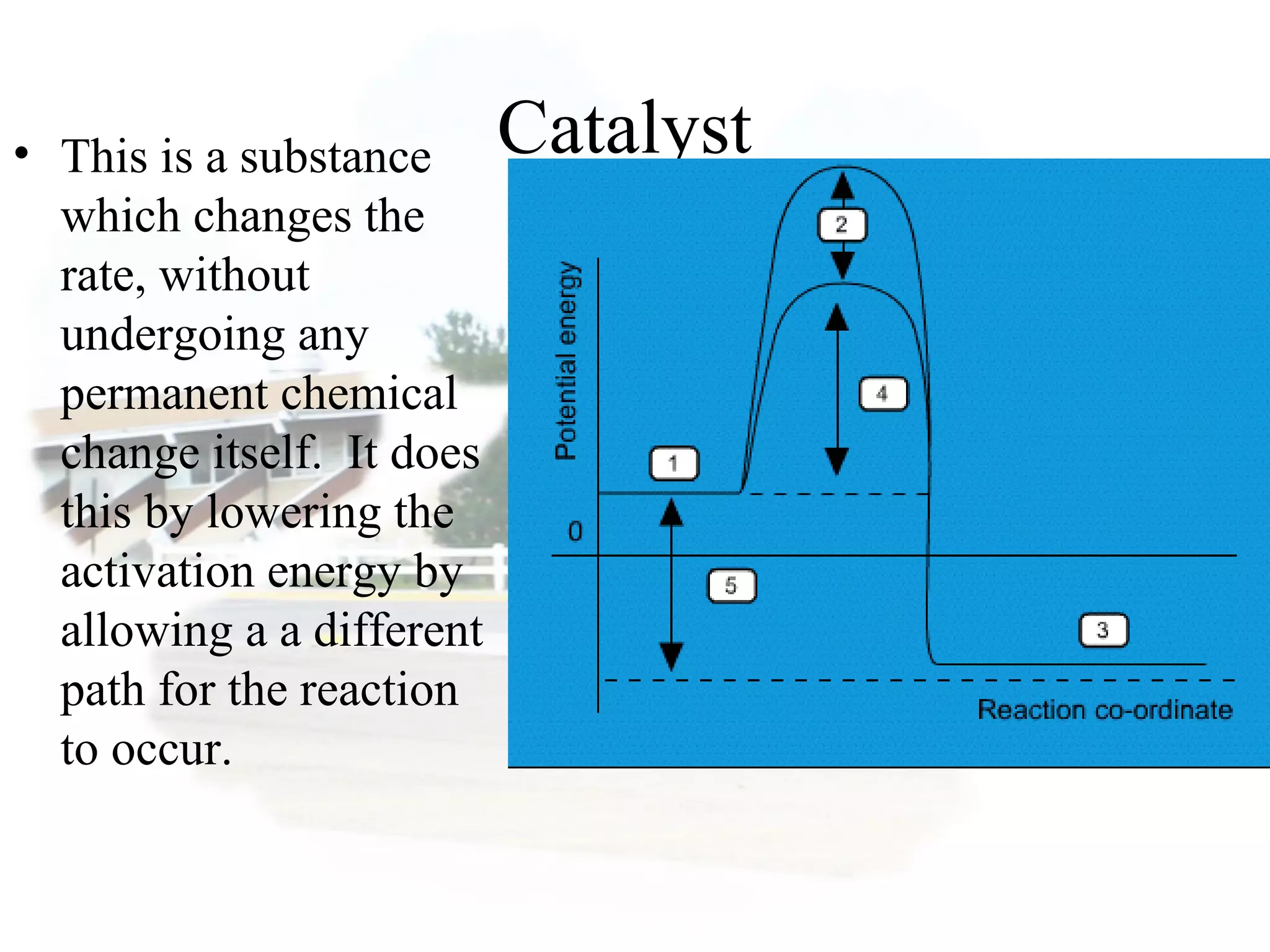
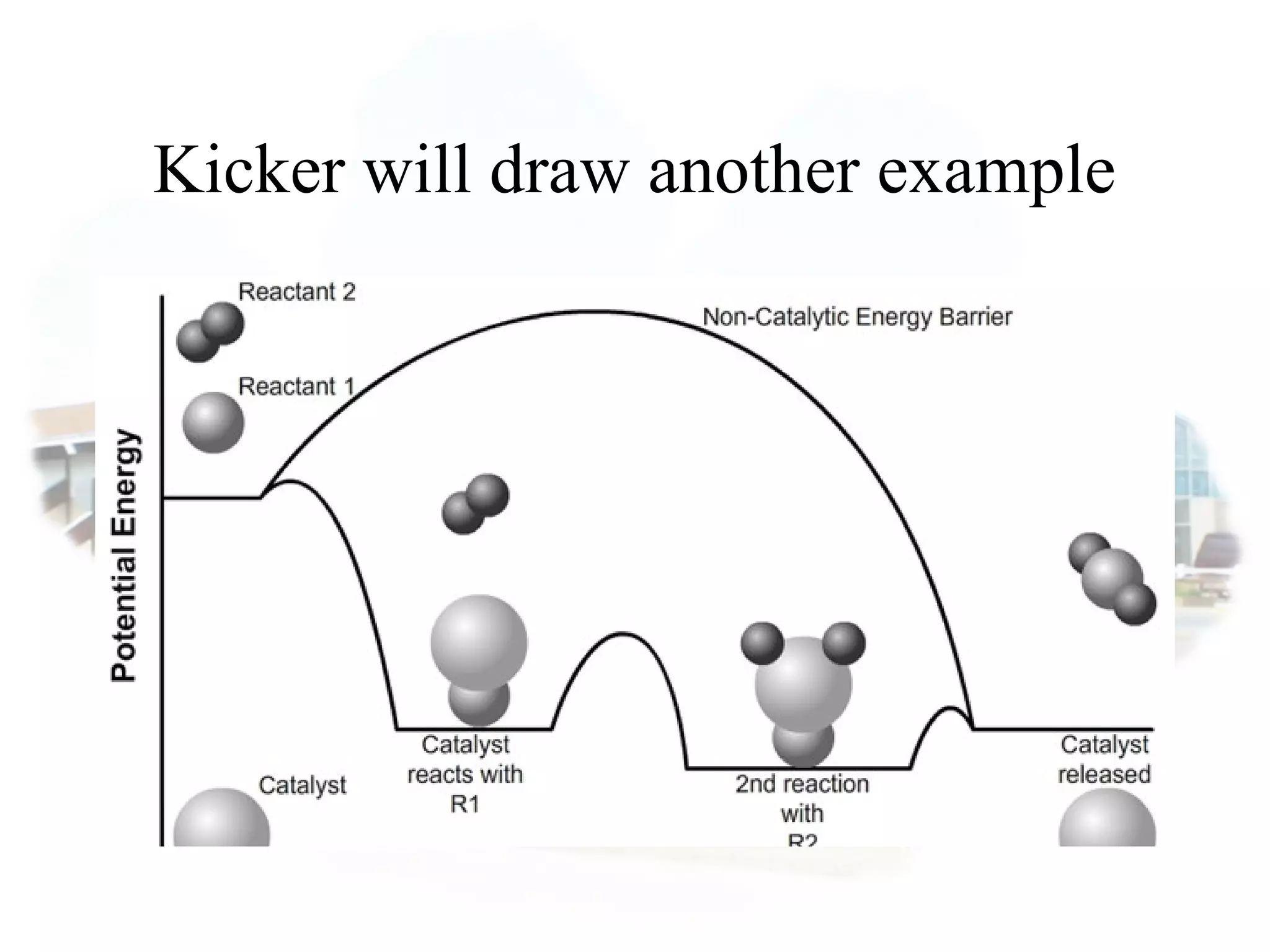
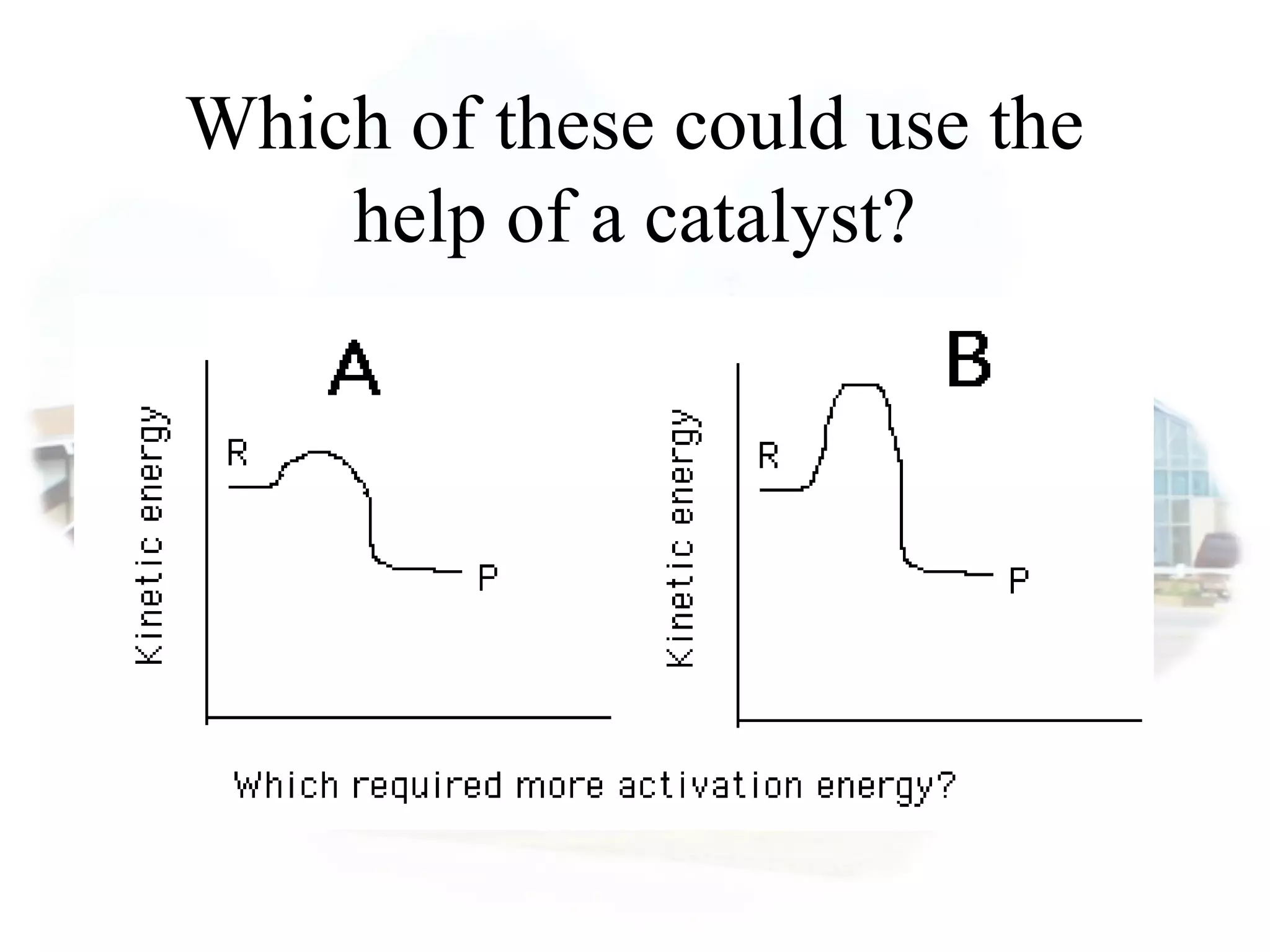
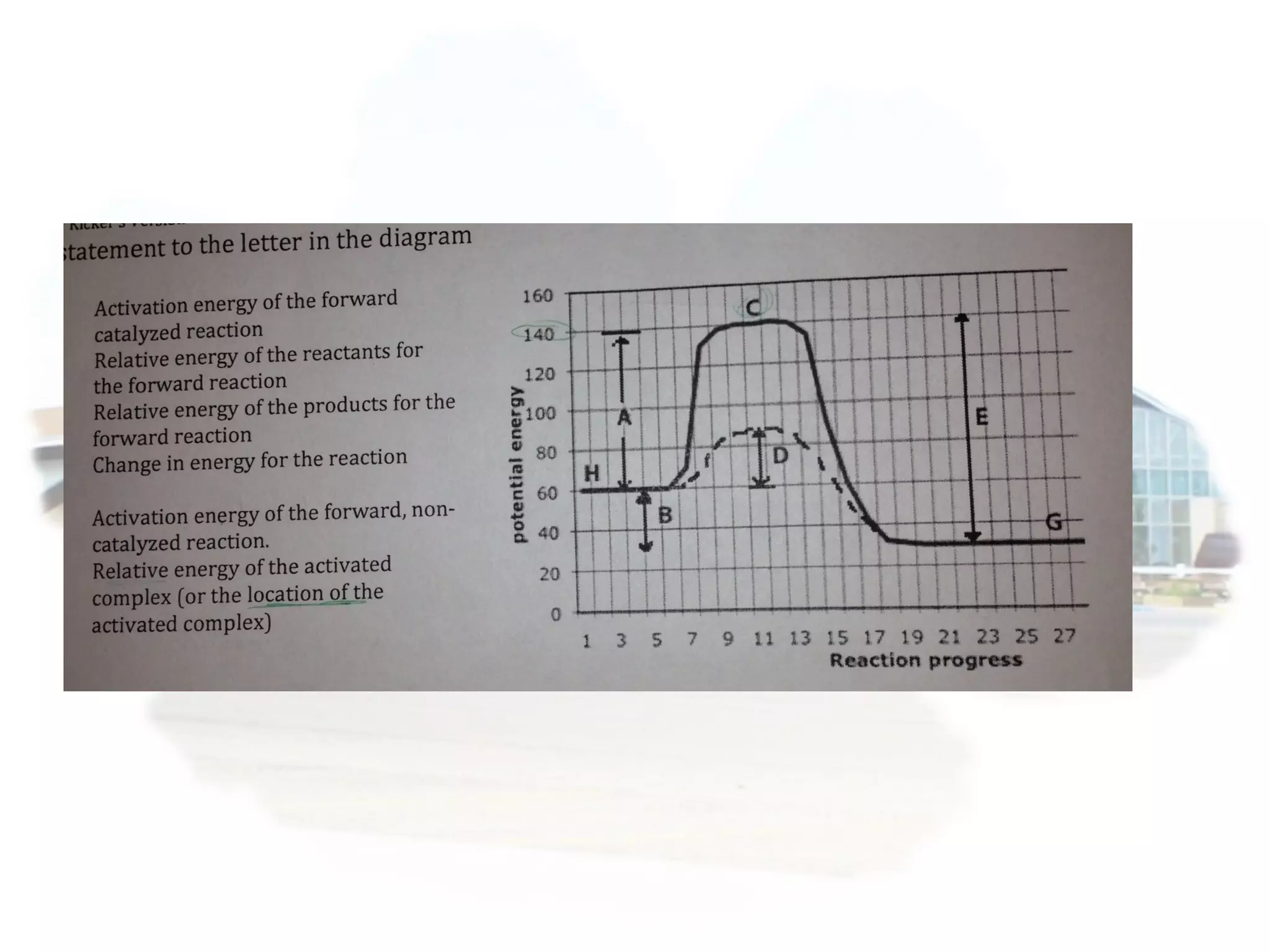
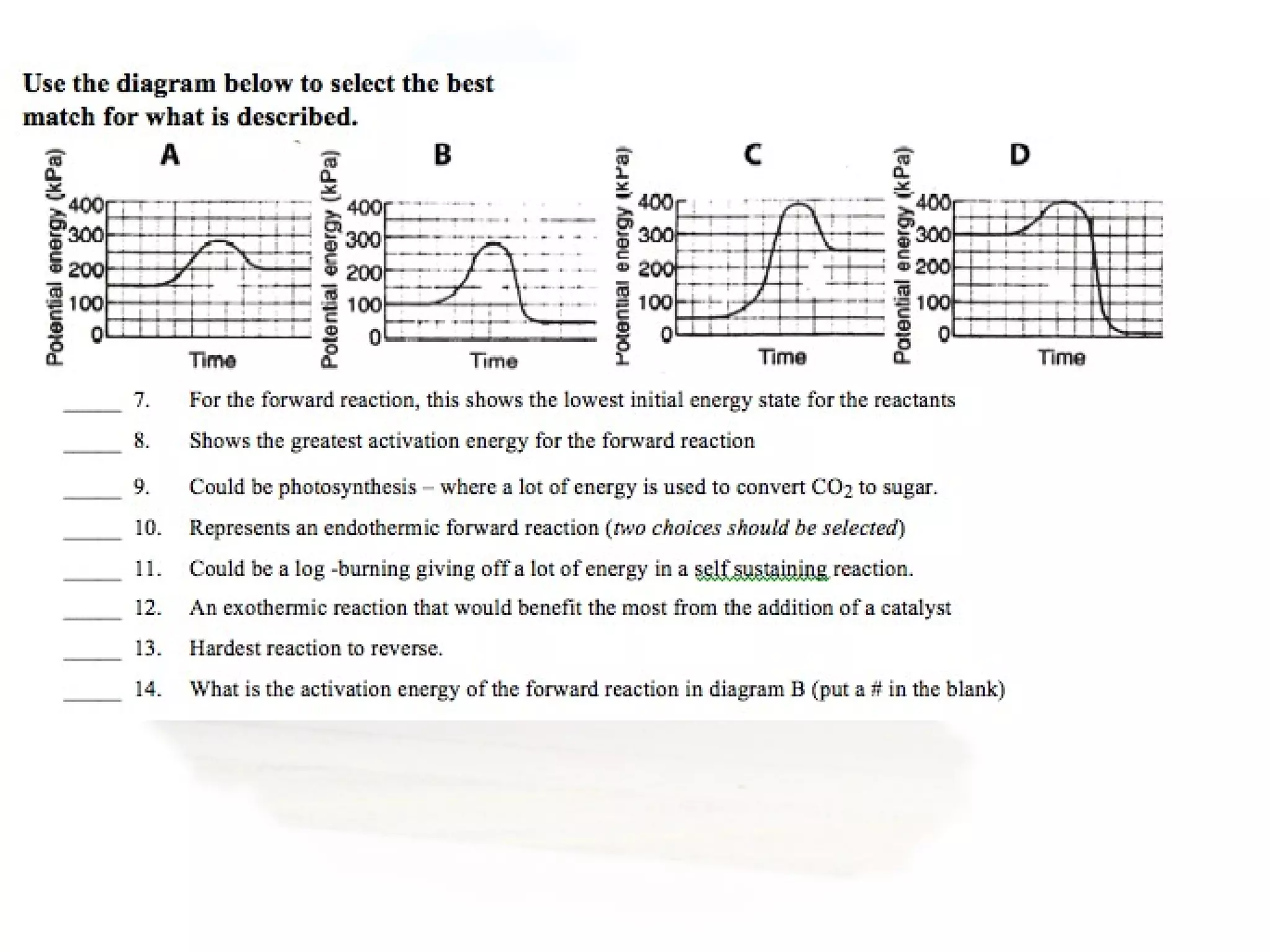
- Collision theory states that reactants must collide with sufficient energy and correct orientation for a reaction to occur, forming an intermediate activated complex.
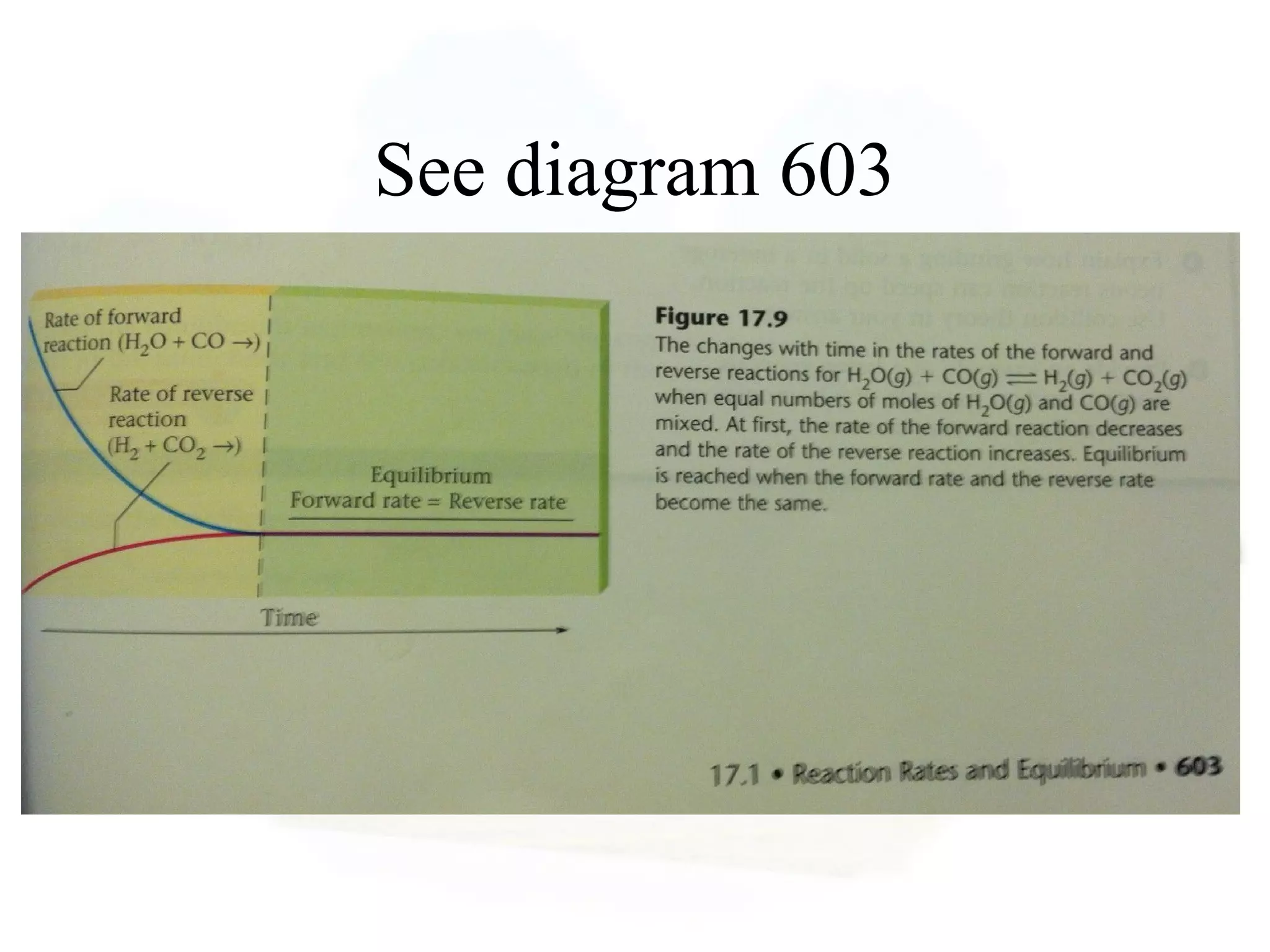
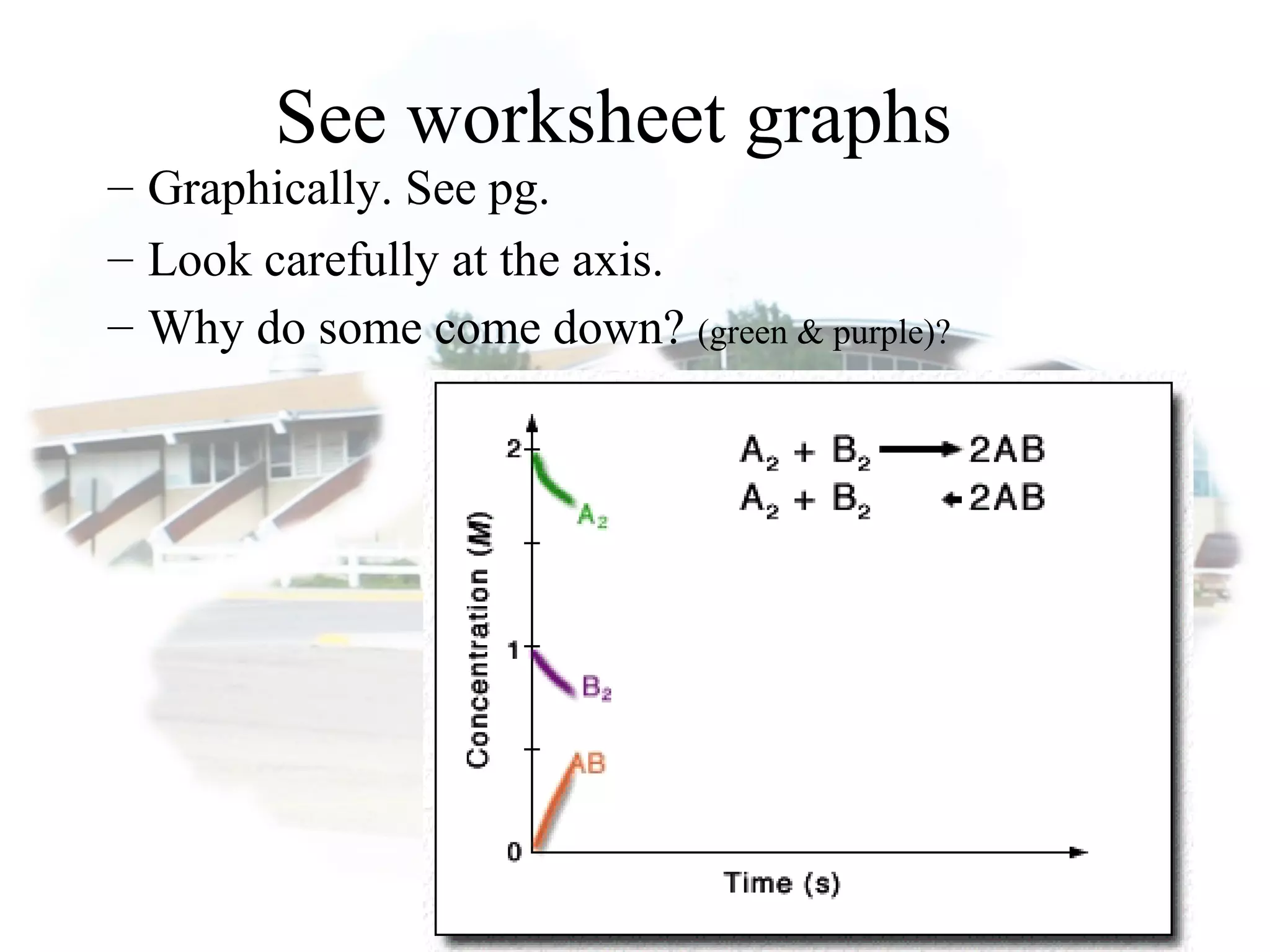
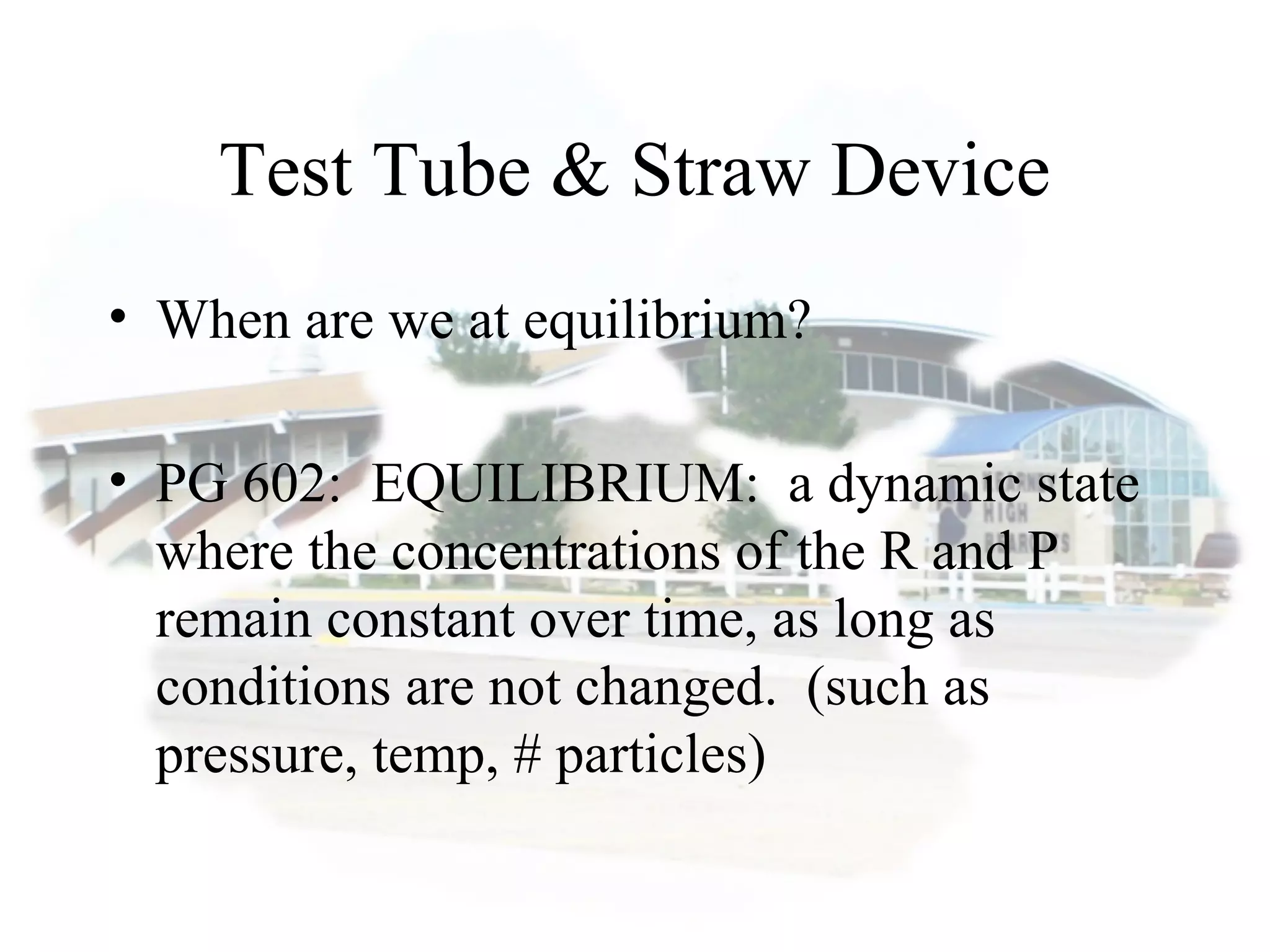
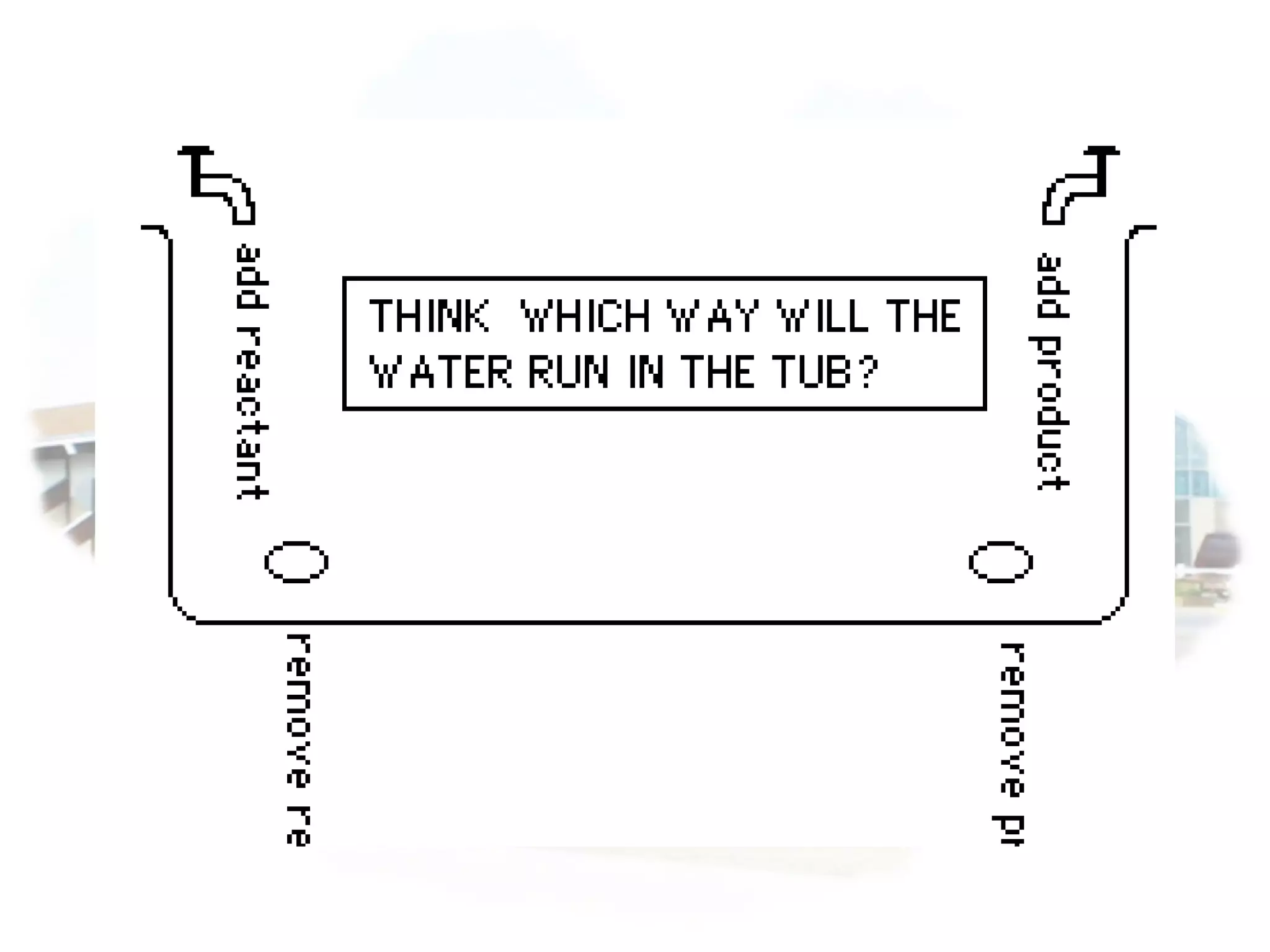
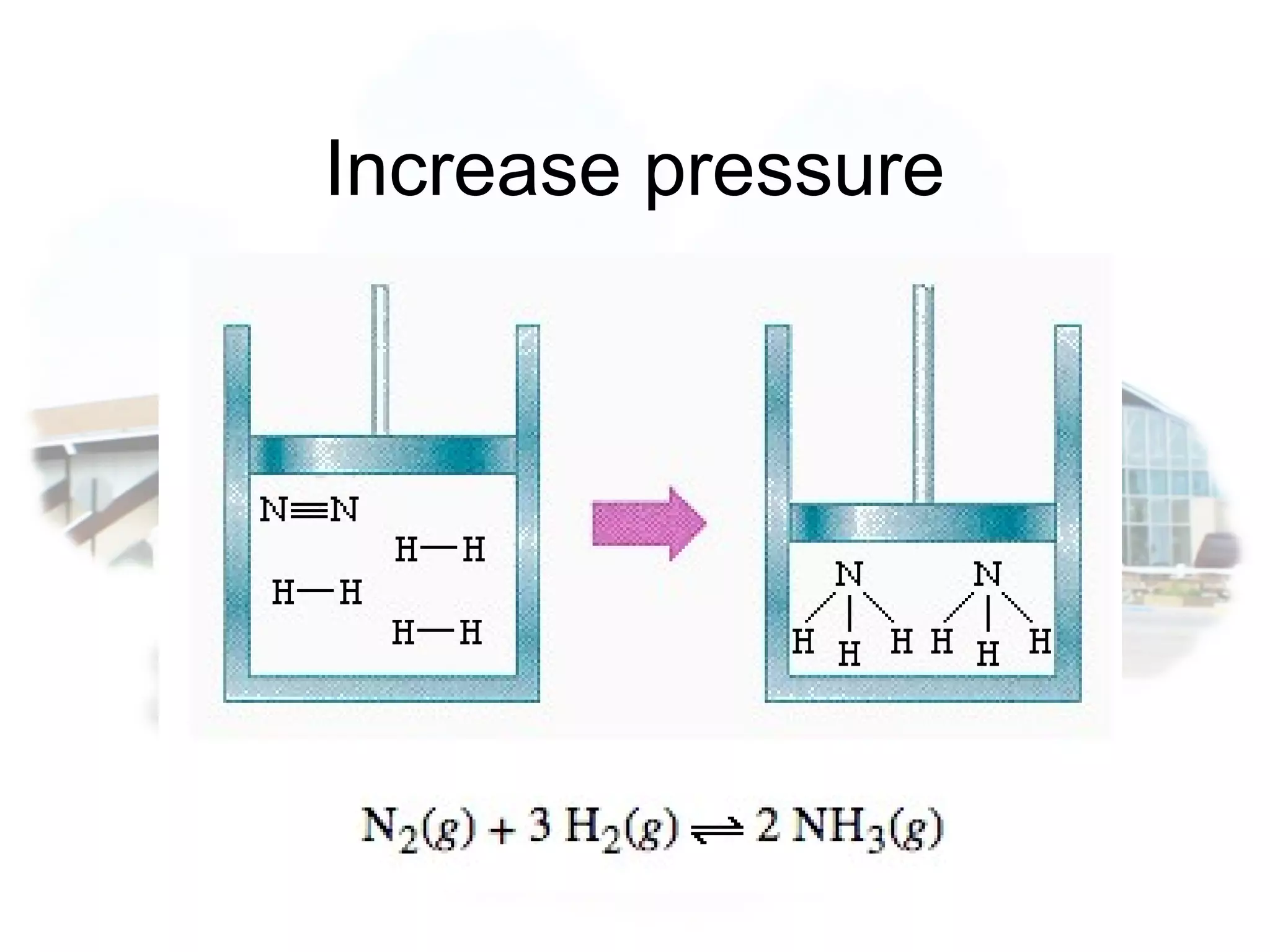
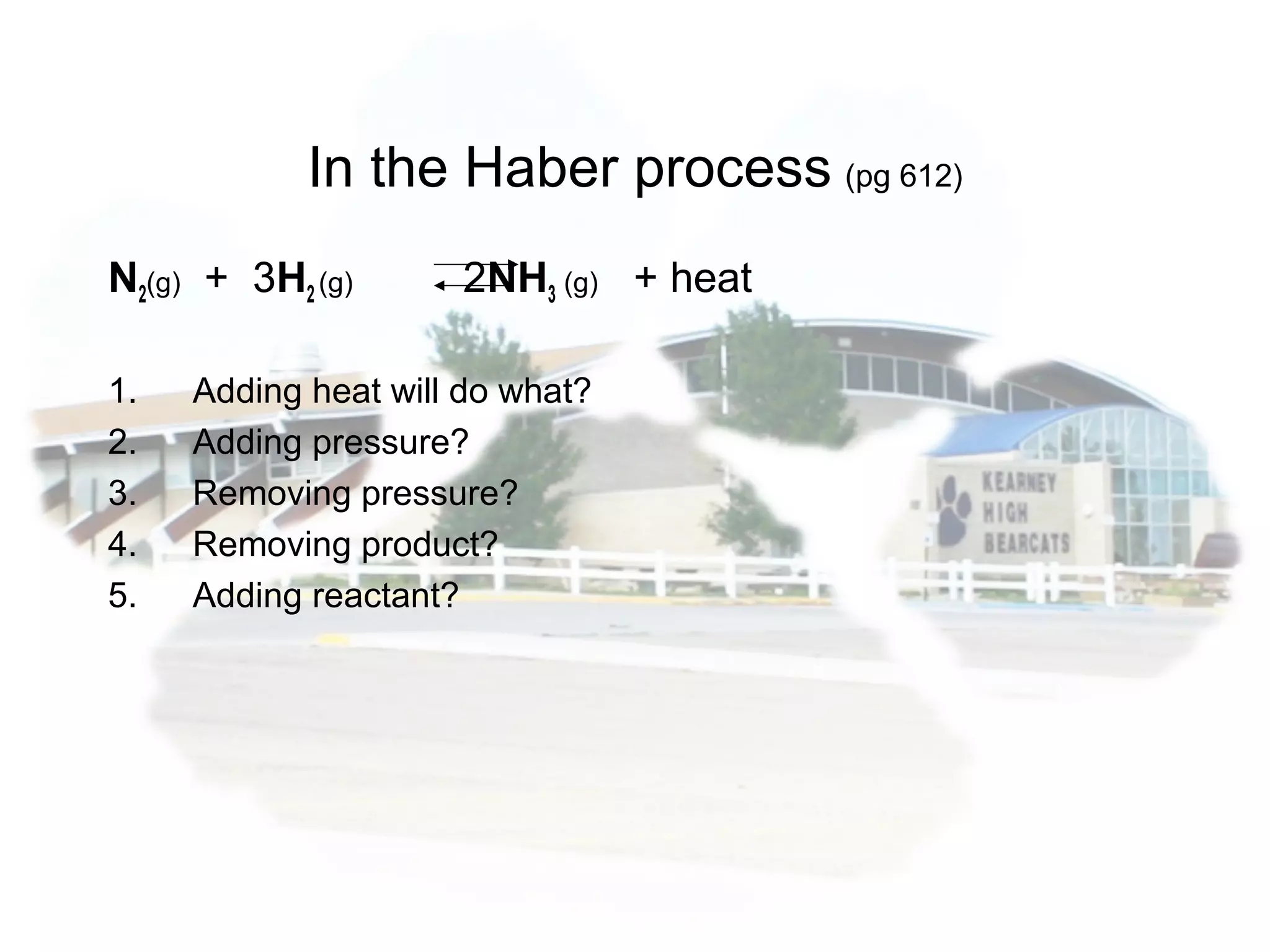
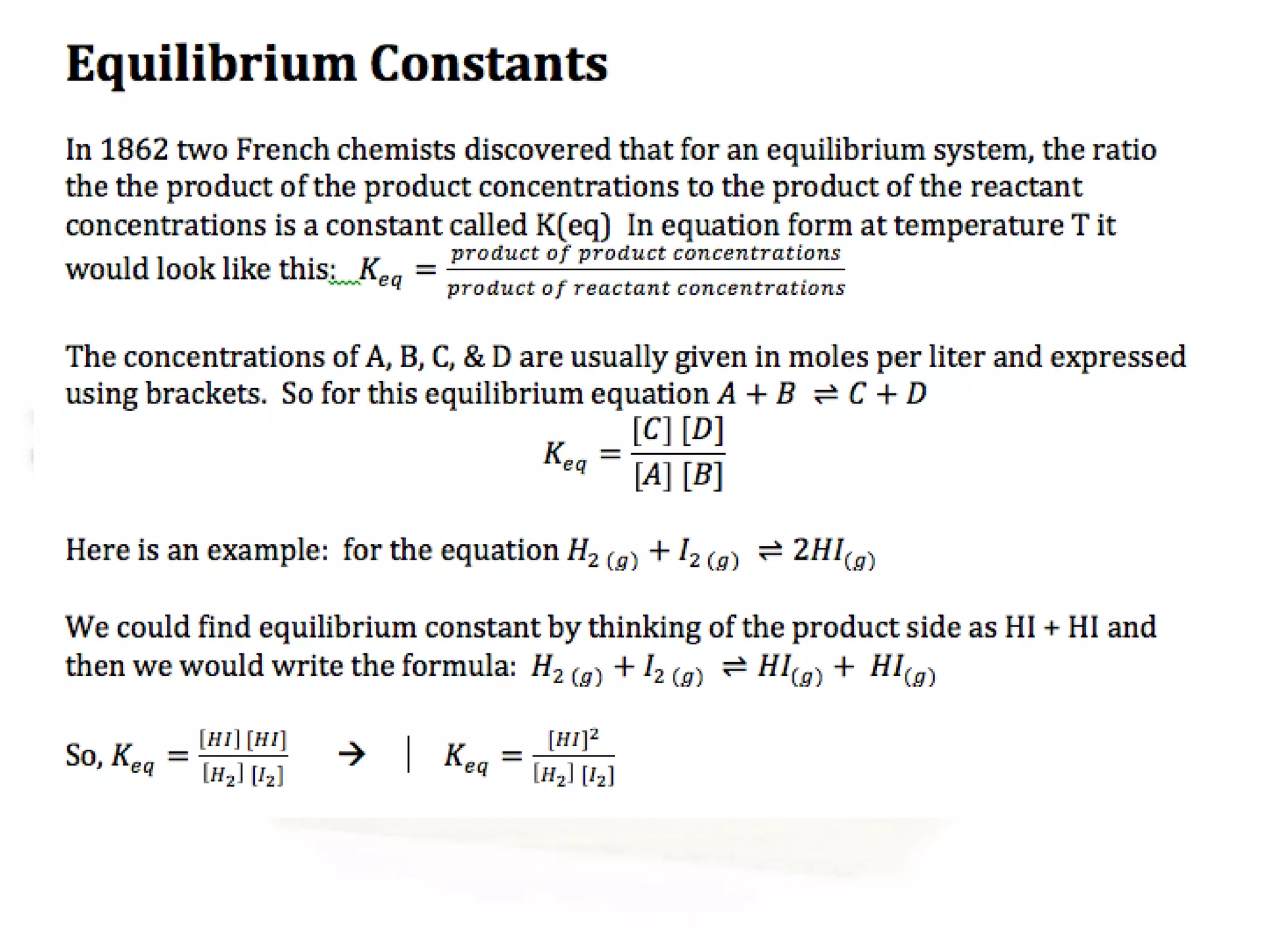
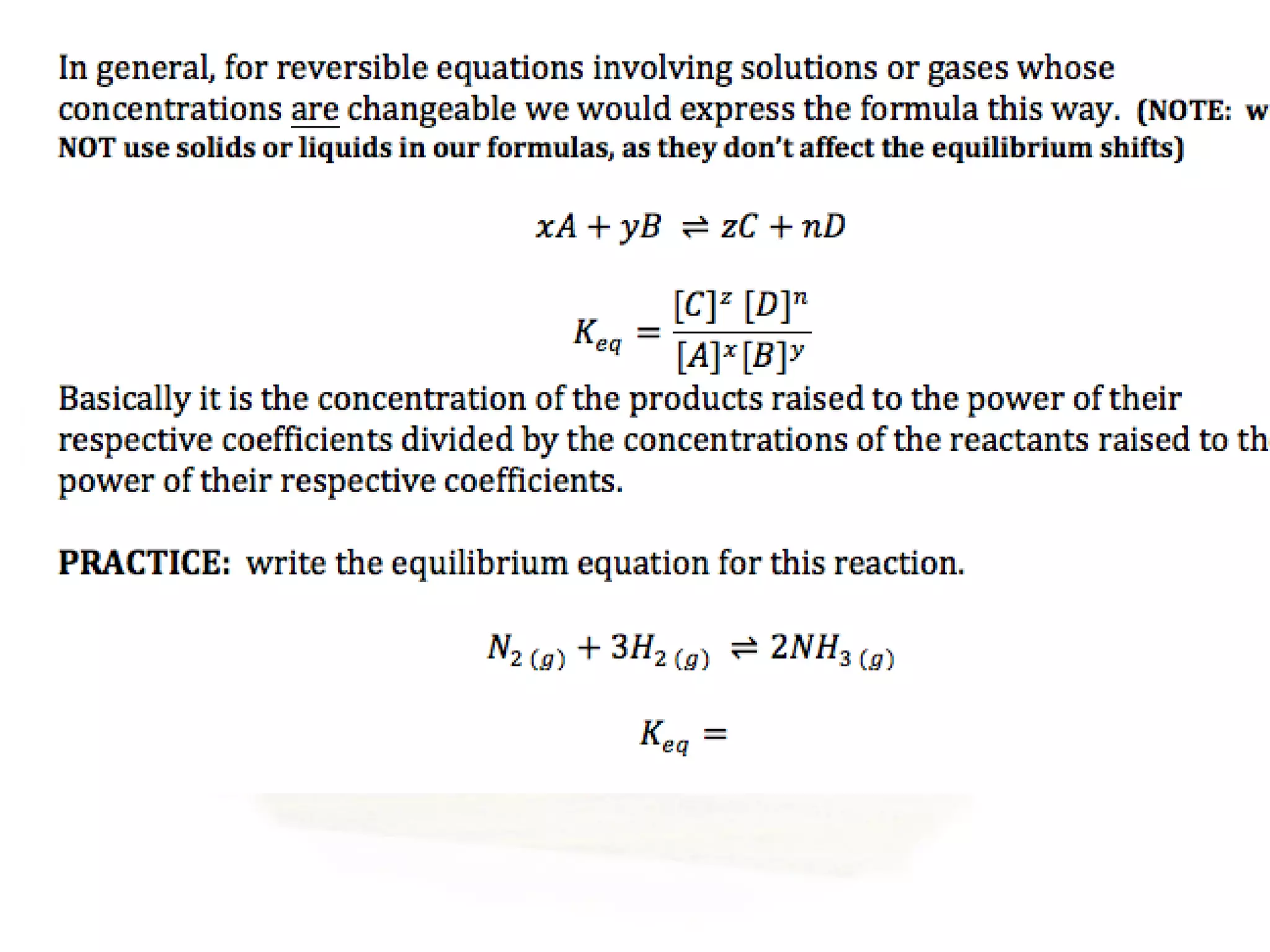
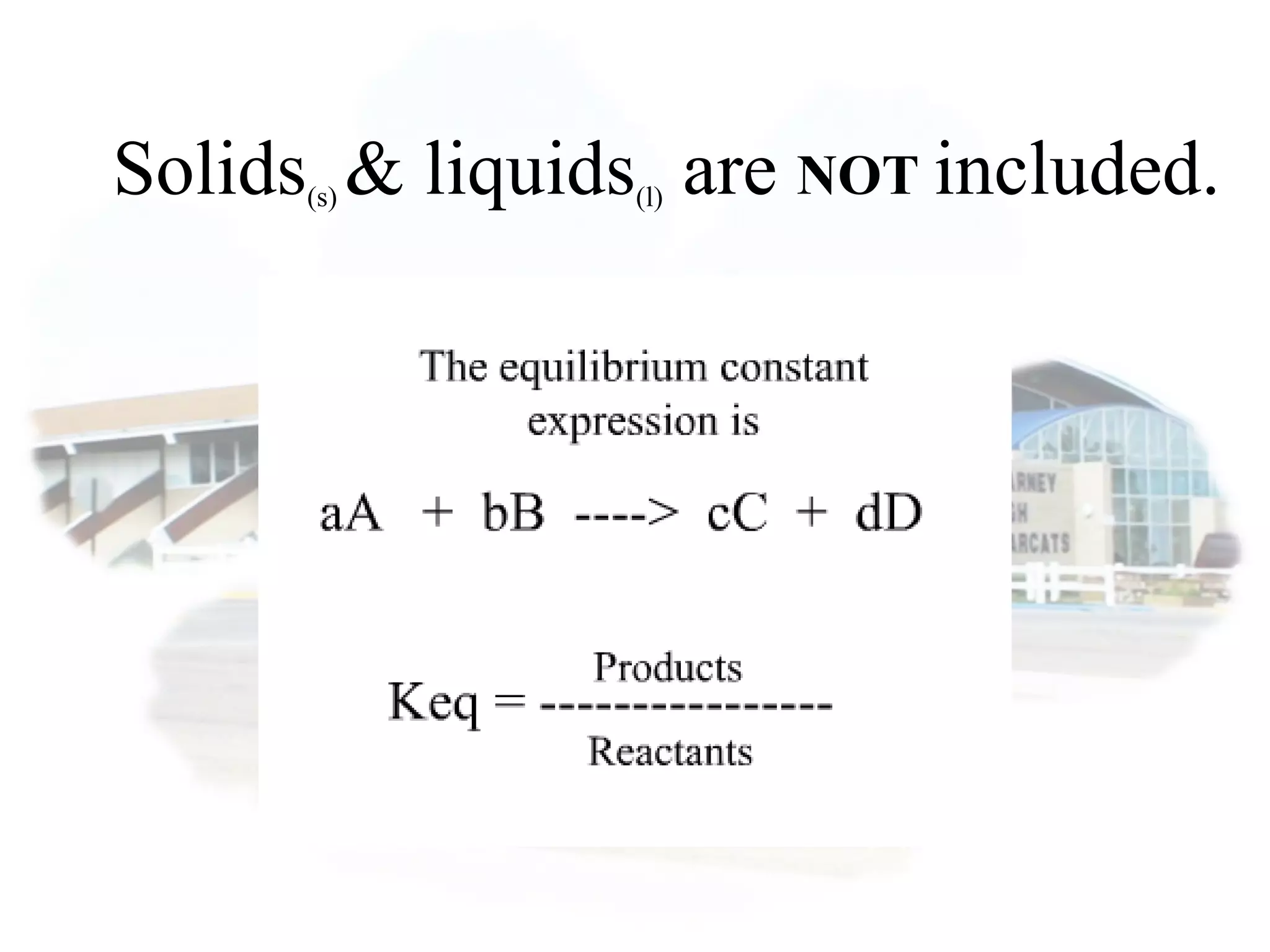
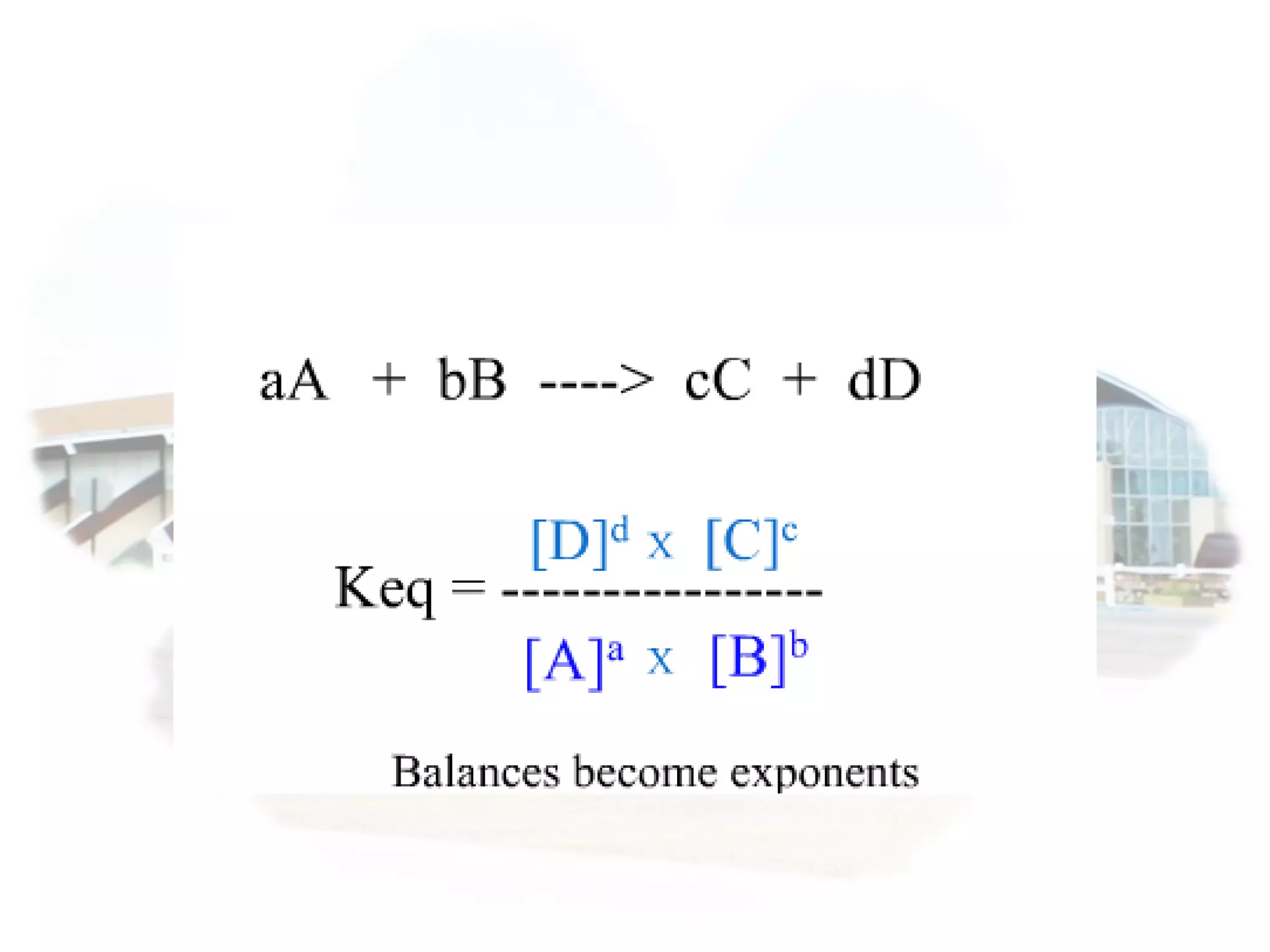
- Equilibrium is reached when the rates of the forward and reverse reactions are equal, though concentrations of reactants and products may not be equal.
-
































![• PRACTICE: 1. Calculate the Keq for
this reaction given the concentrations at a
constant temperature are as follows:
[NO2] = 8.8 M [NO] = 1.2 M [O2] = 1.6 M
2NO(g)+O2 (g) 2NO⇌ 2 (g)](https://image.slidesharecdn.com/unit12rateequilibriumlectures-141204110451-conversion-gate02/75/Unit-12-rate-equilibrium-lectures-63-2048.jpg)

![• PRACTICE: 3. Calculate Keq for the
above reaction (practice 2) if the
concentrations are as follows:
[CuCl2] = 0.050M & [NaCl] = 0.50M
2Na(s)+CuCl2 (aq) Cu⇌ (s)+ 2NaCl(aq)](https://image.slidesharecdn.com/unit12rateequilibriumlectures-141204110451-conversion-gate02/75/Unit-12-rate-equilibrium-lectures-65-2048.jpg)