Embed presentation
Downloaded 87 times
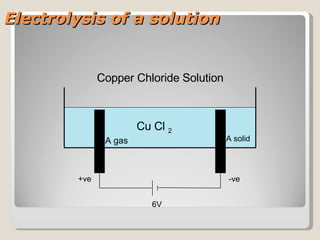

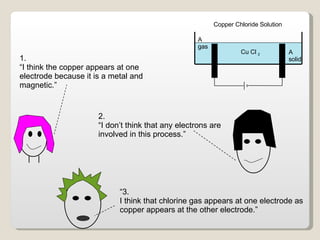


The document describes the electrolysis of a copper chloride solution using a voltage of 6V. During the process, chlorine gas is produced at one electrode while solid copper forms at the other electrode, as electrons are involved in the transfer of copper and chlorine between electrode reactions. Key reactions include the formation of chlorine gas at the anode from chloride ions and copper metal at the cathode from copper ions gaining electrons.




