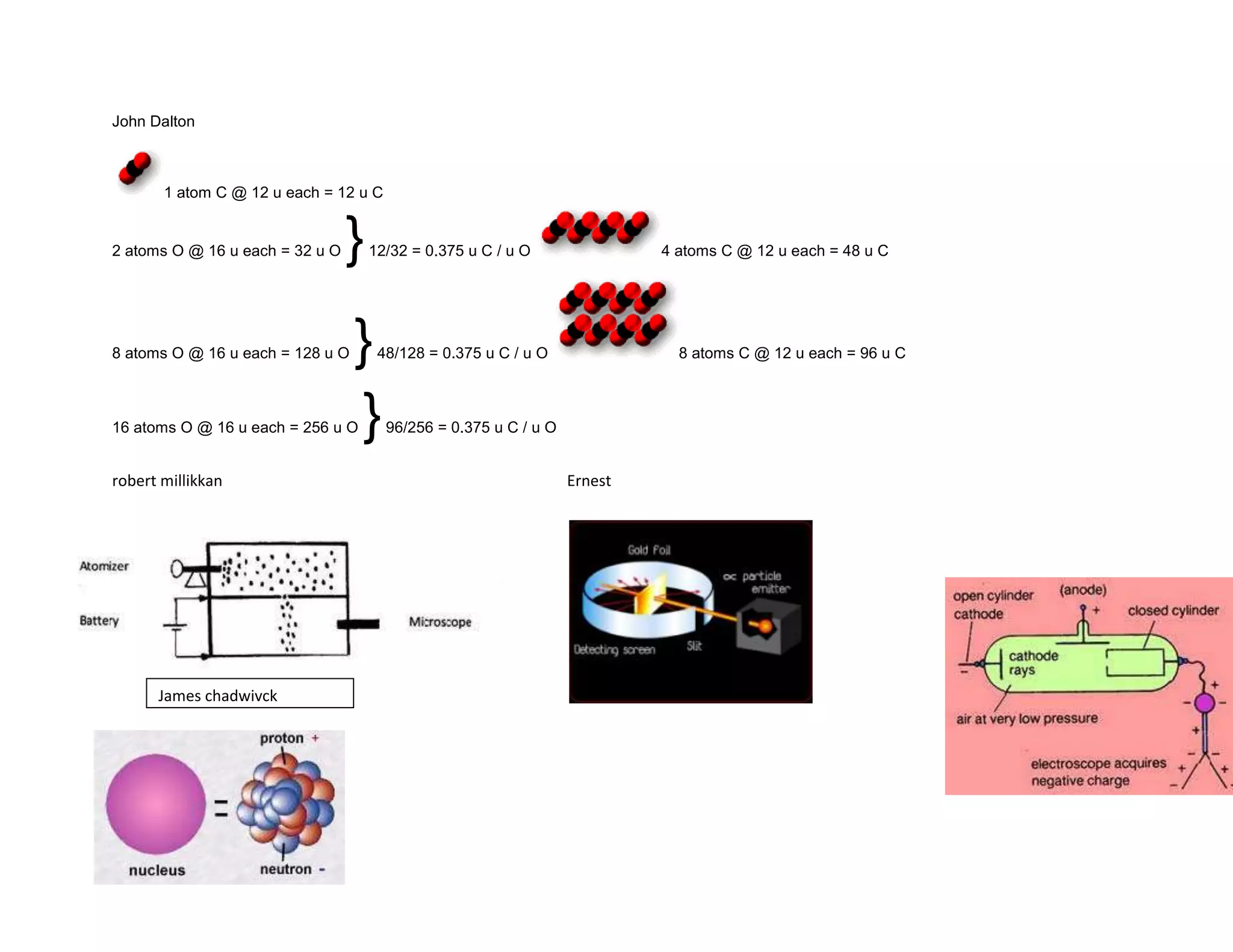
James Chadwick discovered the neutron in 1932. Neutrons were proposed to explain discrepancies between atomic mass and charge. Ernest Rutherford published his atomic theory in 1911 describing the atom as a small, dense nucleus surrounded by orbiting electrons. Most of an atom's mass is in the nucleus, with the rest being empty space. Robert Millikan's 1909 oil drop experiment helped establish the quantized nature of electric charge and supported Thomson's discovery of the electron as a fundamental subatomic particle with a discrete negative charge.