1. The document outlines the history of atomic theory from Democritus to Bohr. It describes early atomic models proposed by Dalton, Thomson, and Rutherford and experiments that led to advances.
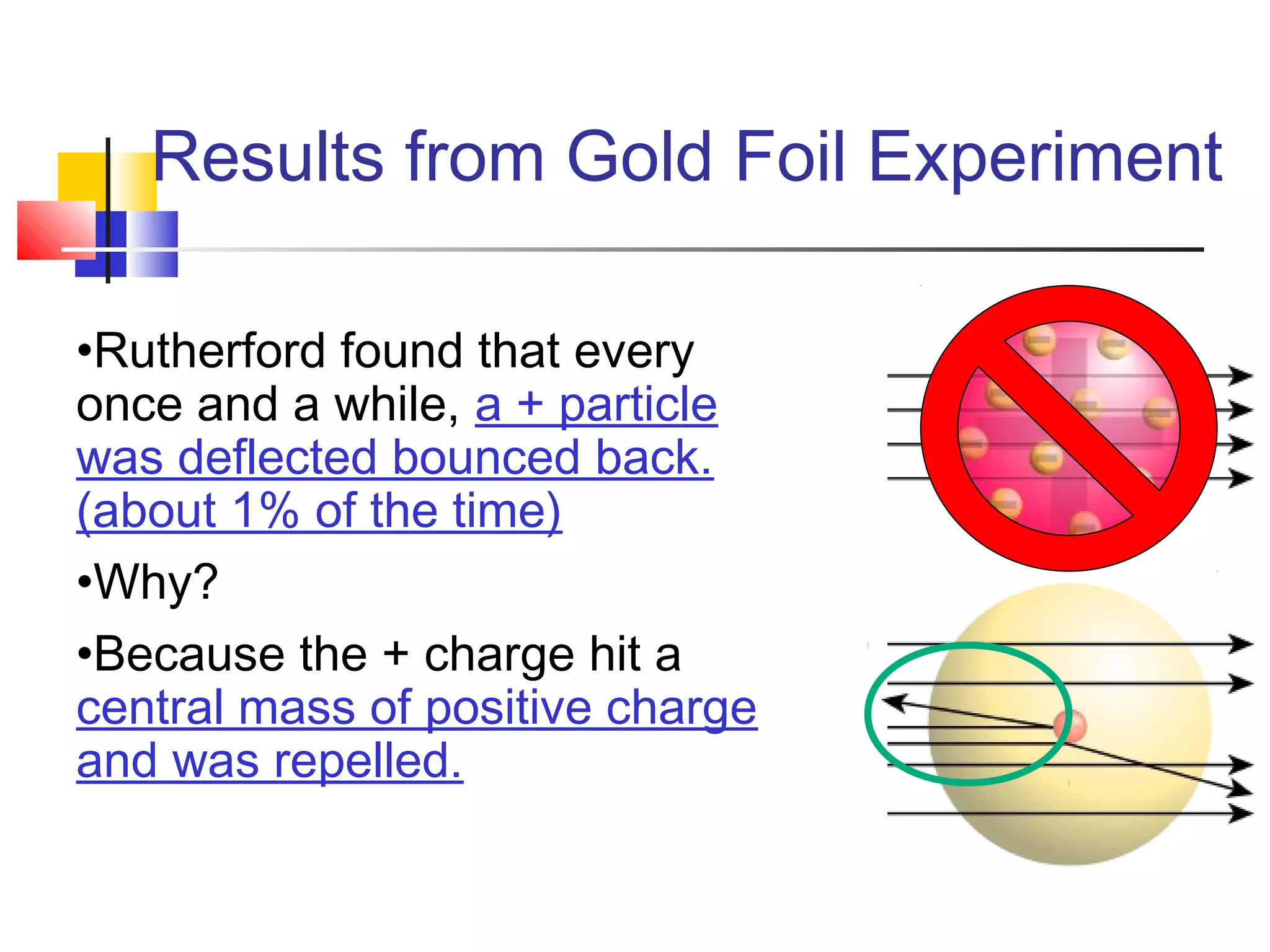
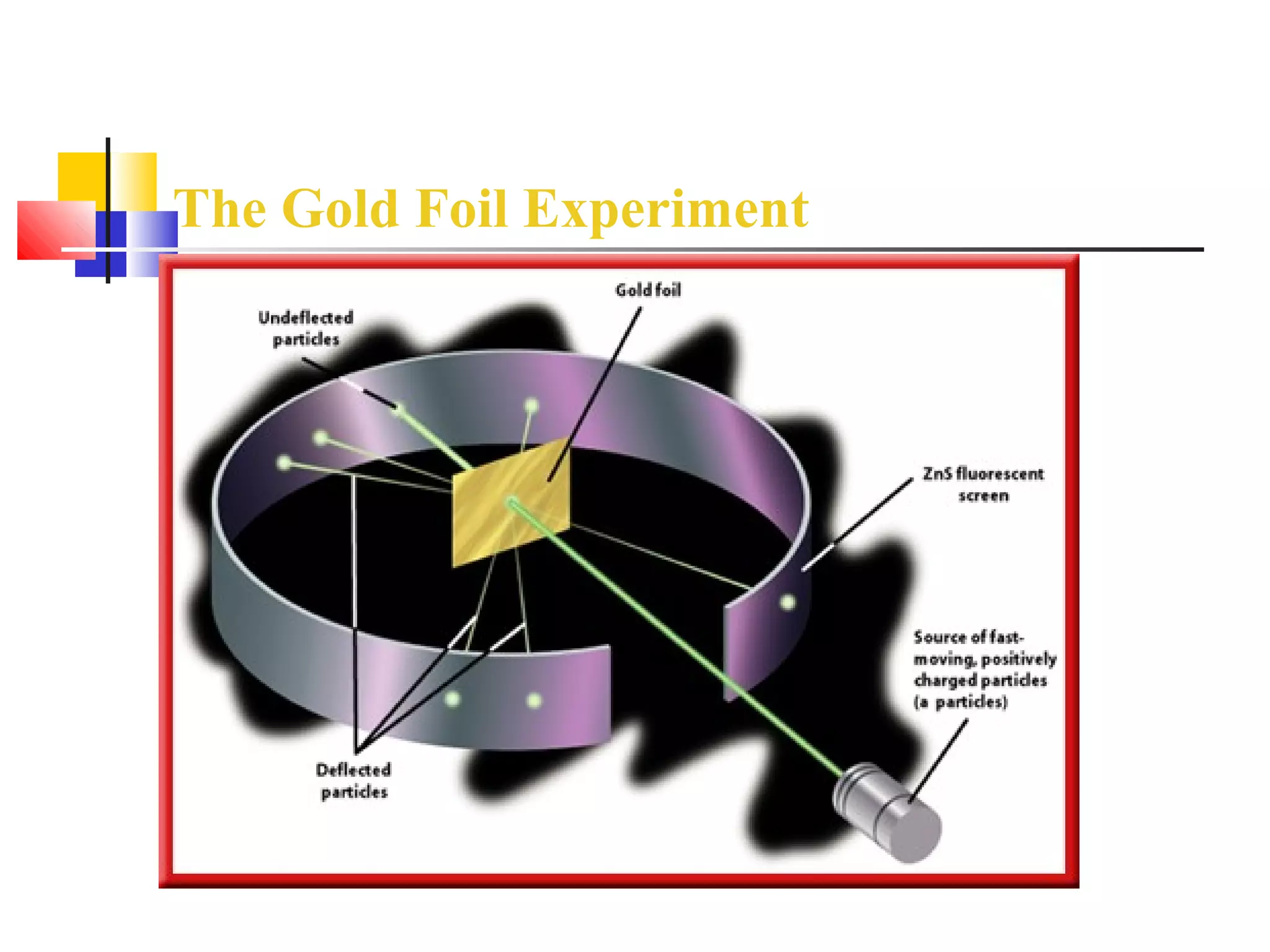
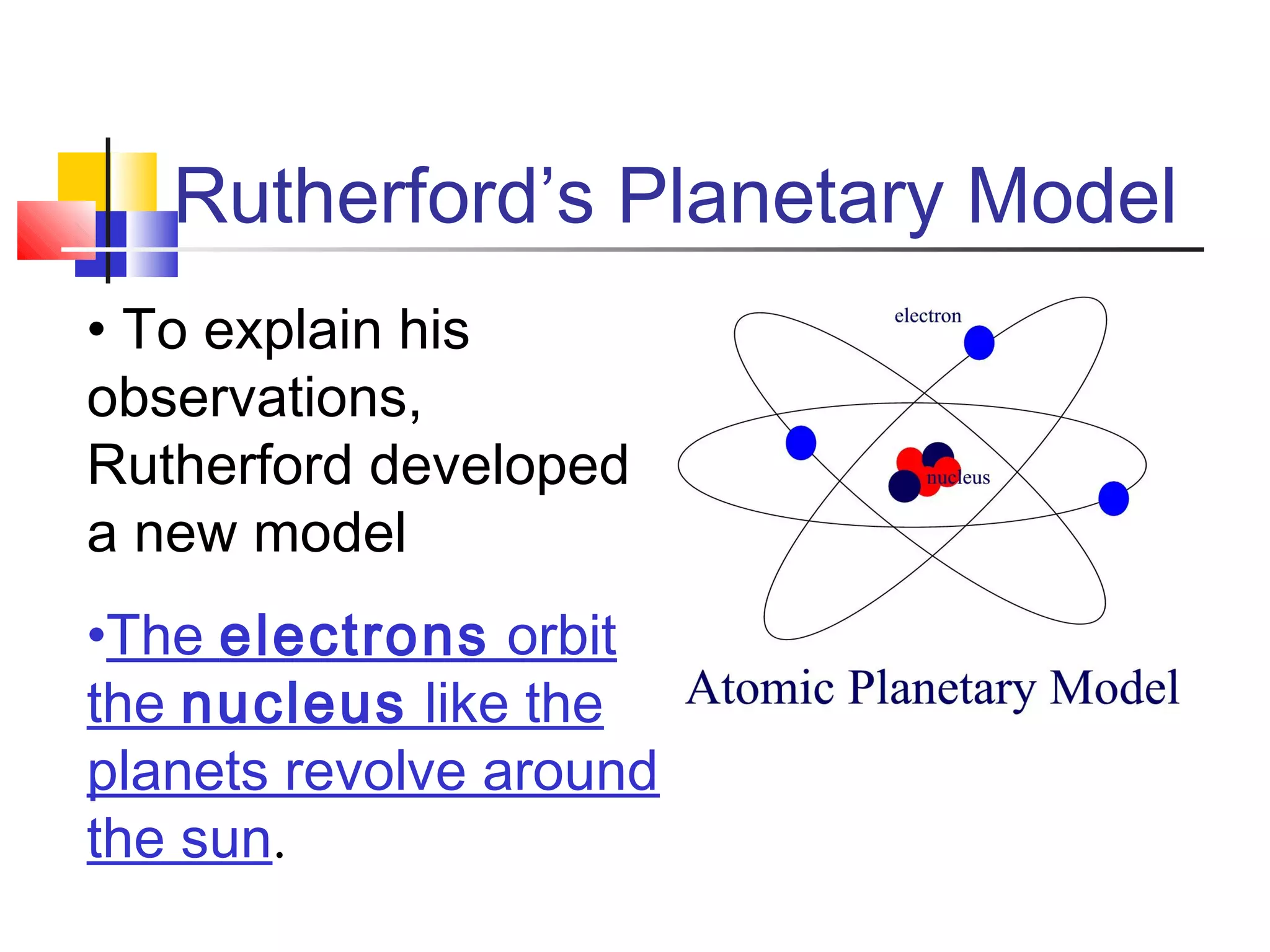
2. Rutherford's gold foil experiment showed that atoms have a small, dense nucleus containing most of their mass.
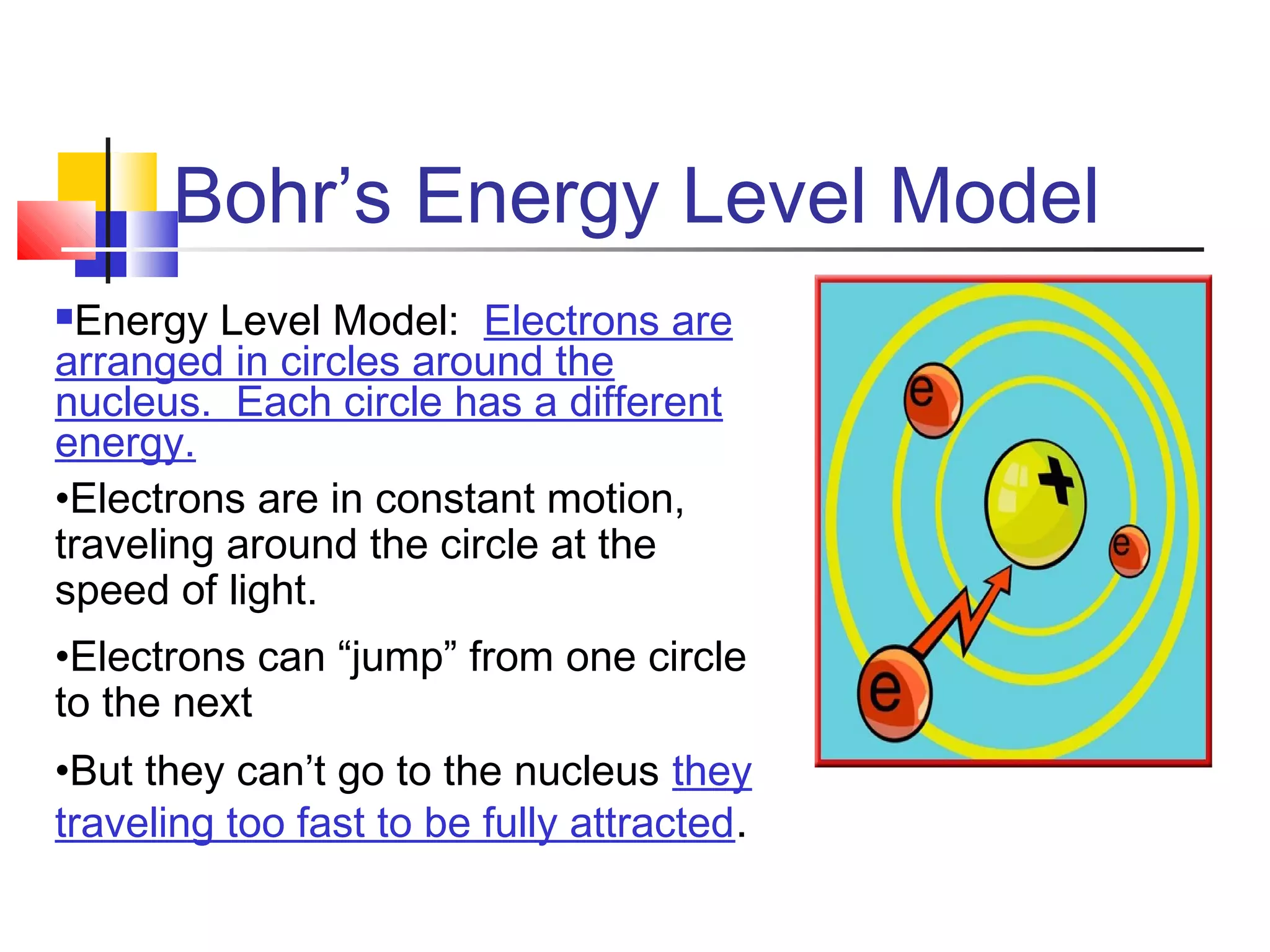
3. Bohr incorporated Rutherford's findings into his model where electrons orbit in fixed energy levels.