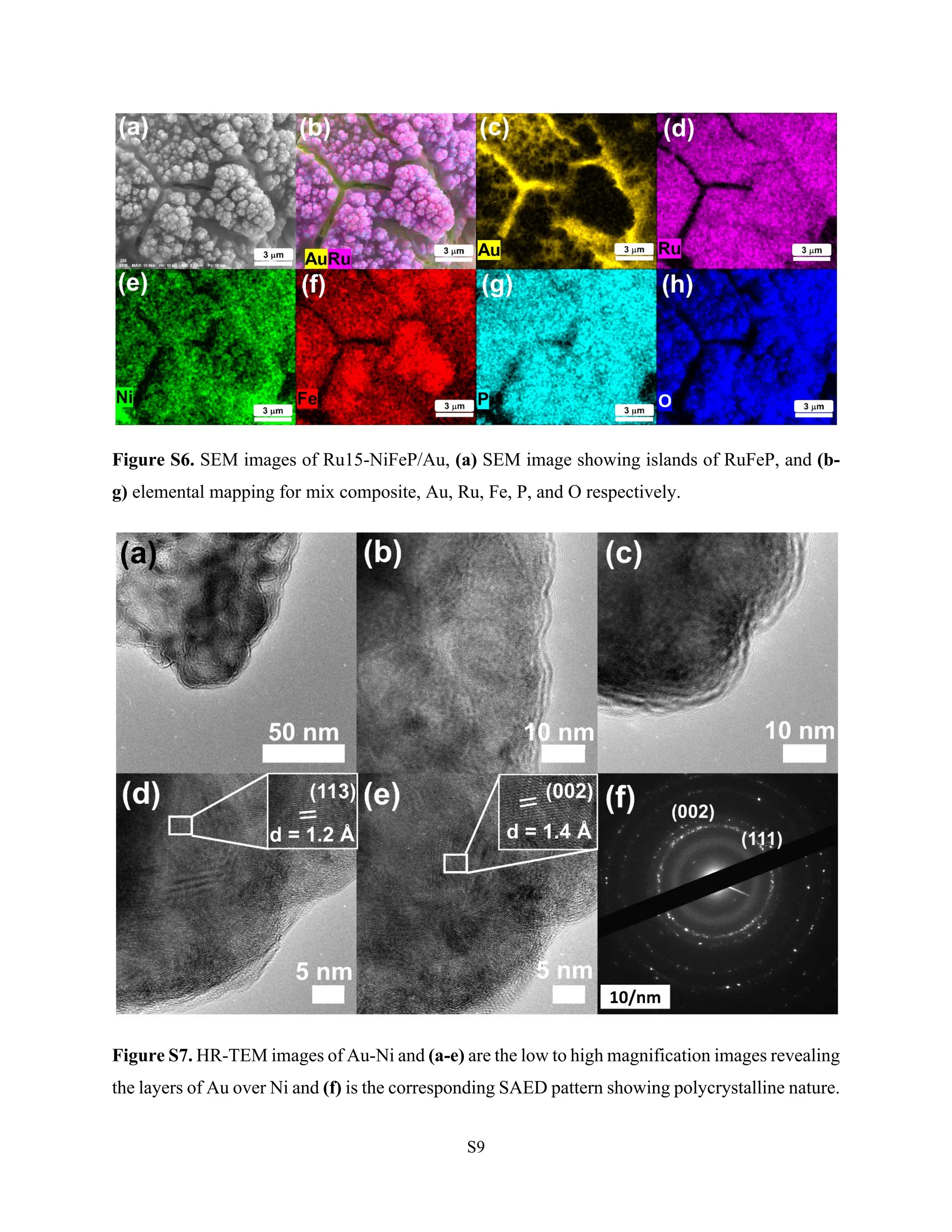
Hydrogen production via electrocatalytic water splitting is largely impeded by anodic oxygen evolution reaction (OER). Herein, we report surface amorphized Ru-NiFeP/Au islands as an effective electrode for OER in 1M KOH reaching a current density of 10 mA cm-2 at 223 mV overpotential. The iR corrected Tafel slope was calculated to be 32 mV/dec while electrochemical impedance spectroscopy (EIS) discerns an explicitly low charge transfer resistance of 0.3 Ω at 400 mV overpotential. The high electrocatalytic activity was attributed to the amorphous nature, reduced band gap, and synergism of Ru-NiFeP with Au. In-situ Surface-enhanced Raman scattering (SERS) reveals the role of FeOOH at lower overpotential for facile OH-adsorption. Evolution of NiOOH peaks at higher overpotential for O2 evolution coupled with synergistic Ru-O bonds to promote OER is studied with DFT analysis. Bader charge analysis evidenced the charge transfer from Fe to O is 0.17 units greater than Ni to O for *OH intermediate generation at active site and corroborates with the results of in-situ SERS studies where FeOOH are active sites at lower overpotentials. The bond order characteristics is pronounced when FeOOH/NiOOH surfaces are accessible. DFT analysis revealed the low free energy change (0.12 eV) for the rate-determining step at RuO/NiFe-OOH surface.


























![S28
type-2 surface sites and, on the type-1 and type-2 RuO/NiFe-OOH at Ru, Then, the adsorption
energy of intermediates involved are calculated using the following equations:
ΔEads(OH) = E(*OH) − [E* + E(H2O) − 0.5E(H2)] (1)
ΔEads(O) = E(*O) − [E* + E(H2O) − E(H2)] (2)
ΔEads(OOH) = E(*OOH) − [E* + 2E(H2O) − 1.5E(H2)] (3)
ΔEads(OH), ΔEads(O) and ΔEads(OOH) are the adsorption energies of *OH, *O and *OOH
intermediates, respectively. E(*OH), E(*O) and E(*OOH) are the total energy of the system
(surface + intermediate), E* is the energy of the surface, E(H2O) and E(H2) are the energy of the
reference molecules. To further analyze the thermodynamics of the reaction, the calculated
adsorption energies were used to obtain the Gibbs free energies using the following equation24:
ΔG = ΔEads + ΔZPE − TΔS + ΔGU (4)
The Gibbs free energy for OER reaction is given as [6]:
ΔG = ΔEads + ΔZPE − TΔS - eU (5)
Here, ΔZPE is the difference in zero-point energies of the species, and e represents number of
electrons transferred. Only vibrational energy contribution was considered during the calculation
of entropies (Table S5-S8). The zero-point energy calculations were performed using vaspkit.25
ΔGU represents the free energy correction due to electrode potentials. The temperature was taken
to be 298.18 K.
Gibbs free energy for all the steps (Eq 1-4) are ΔG1, ΔG2, ΔG3 and ΔG4 , respectively. The
potential limiting step is given as,
ΔGPLS = max {ΔG1, ΔG2, ΔG3, ΔG4} (6)
The limiting potential,
UL = -
∆𝐺𝑃𝐿𝑆
𝑒](https://image.slidesharecdn.com/d5ta00958h1-250509010427-5f7118d8/75/Surface-Amorphized-in-situ-RuO-NiFeOOH-Au-Islands-for-Electrocatalytic-Oxygen-Evolution-Reaction-28-2048.jpg)










